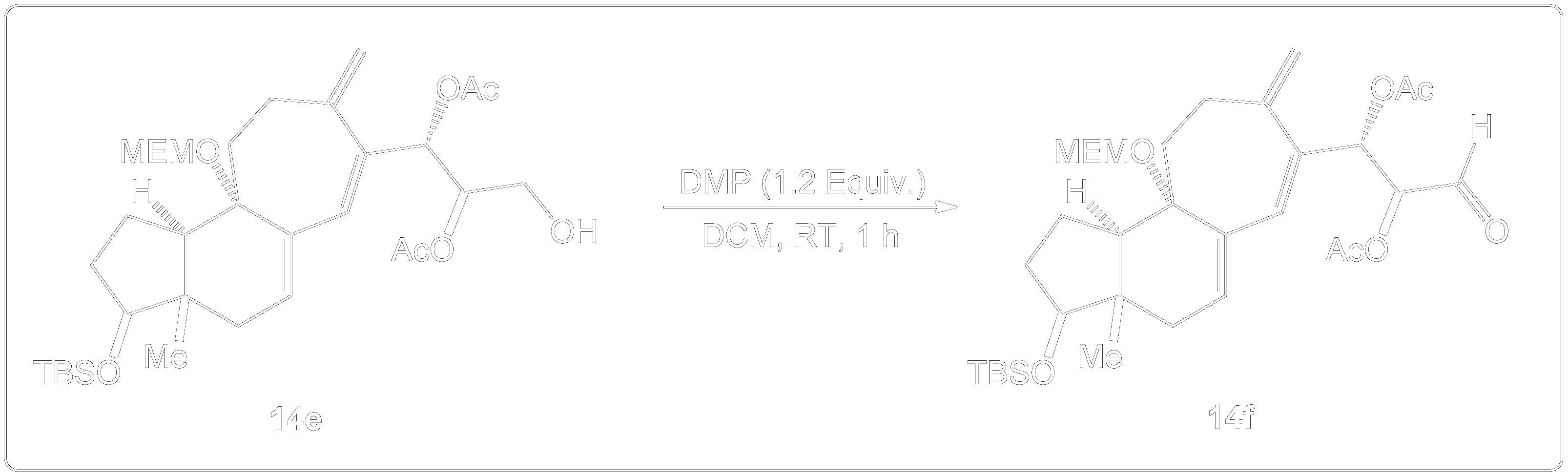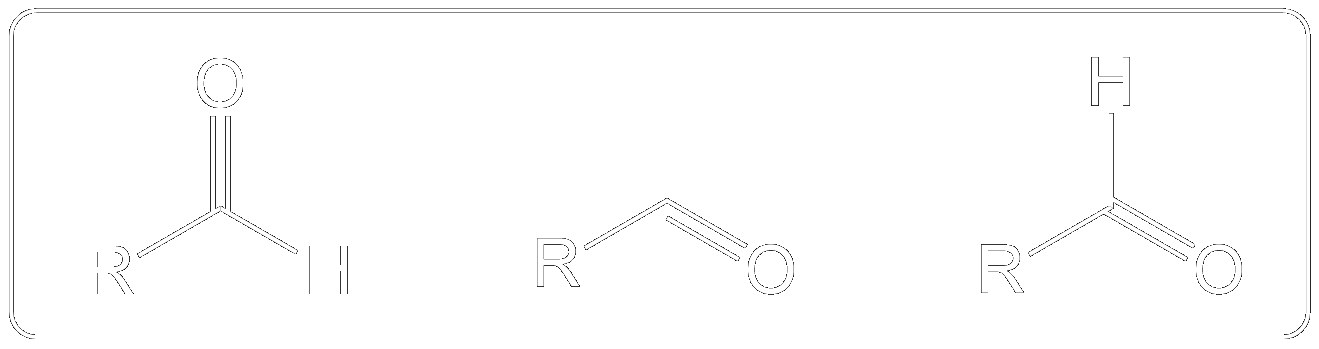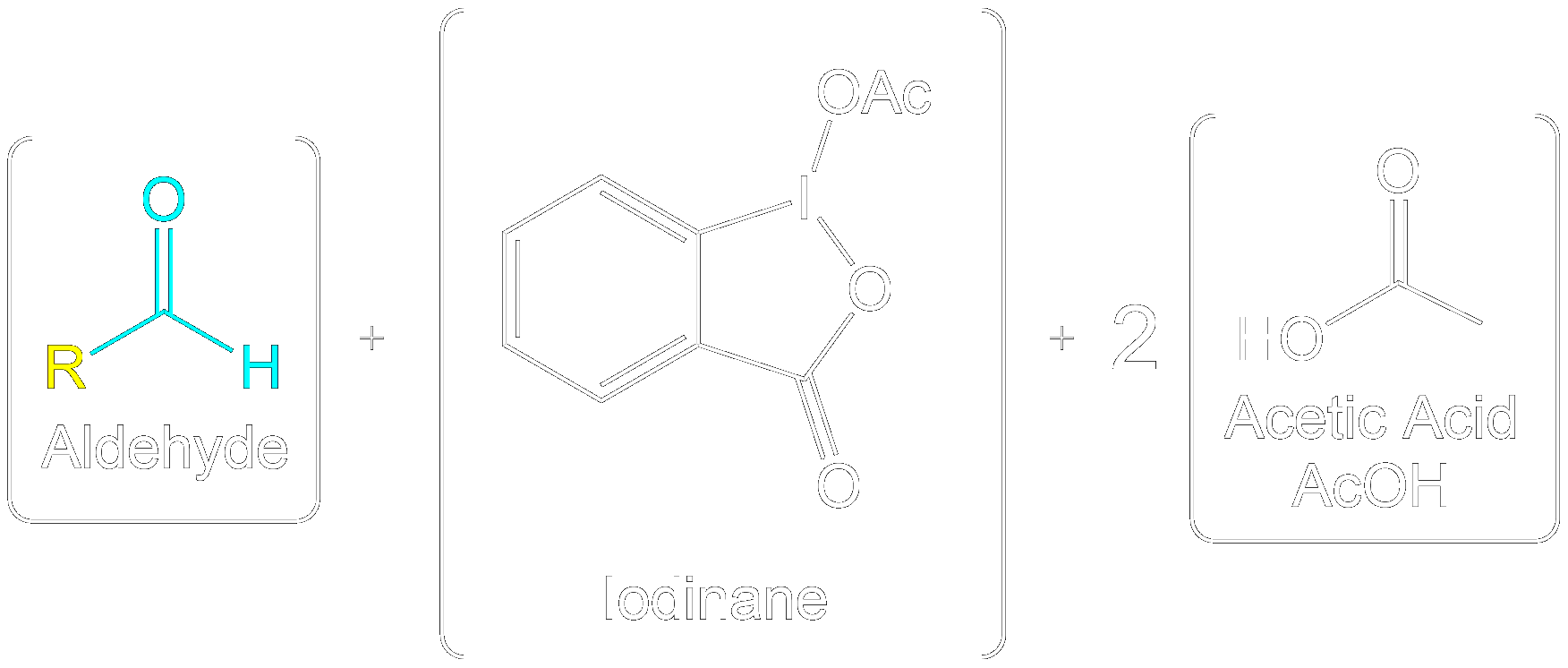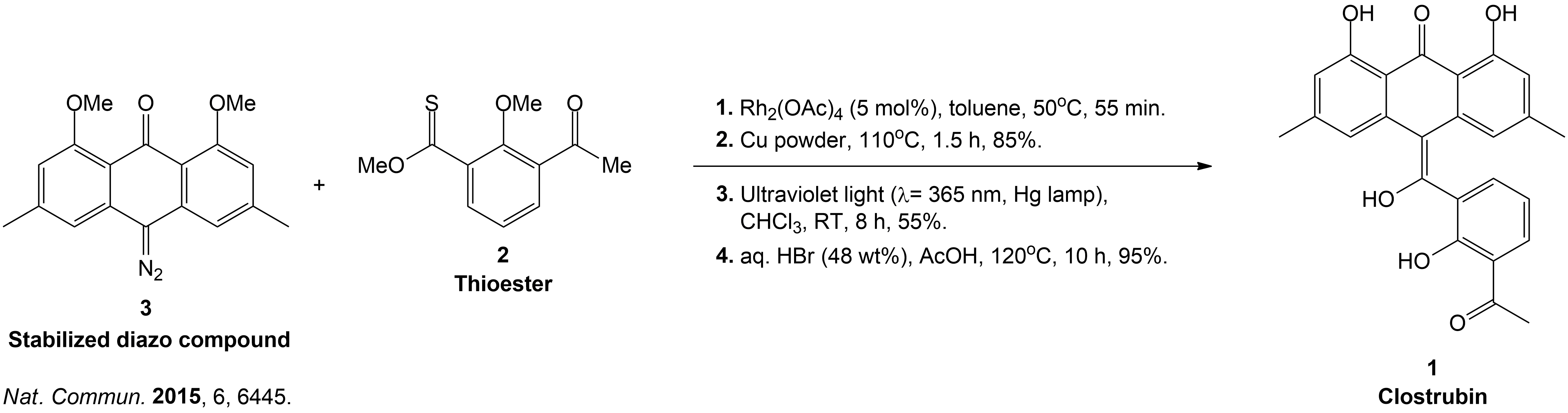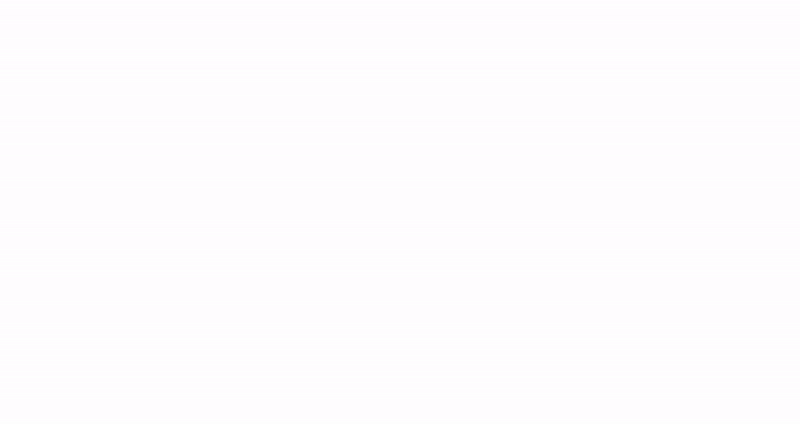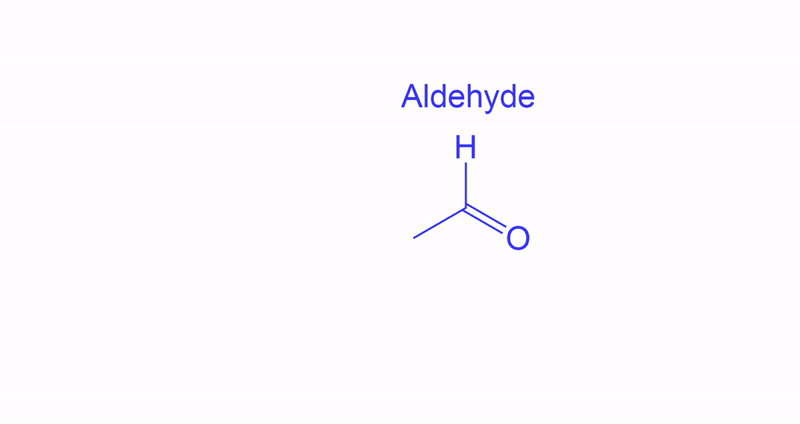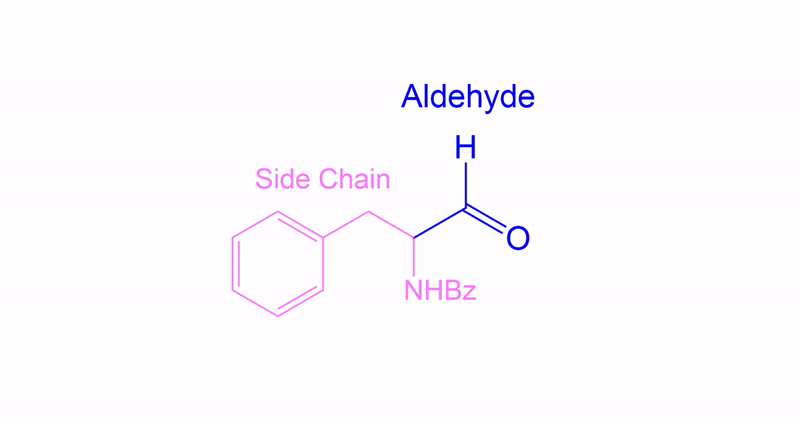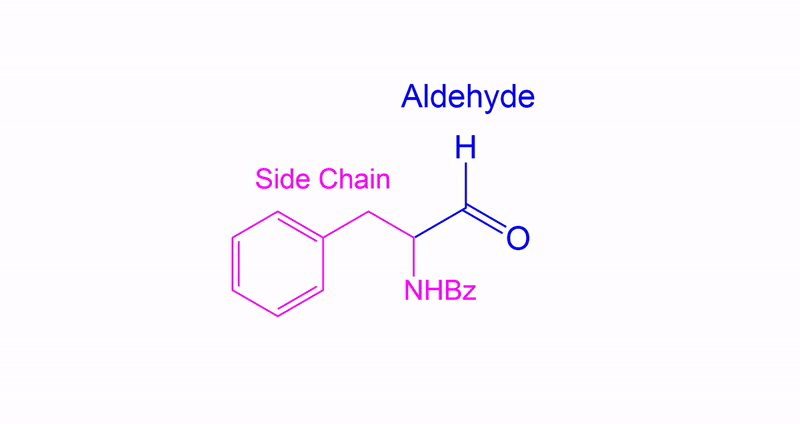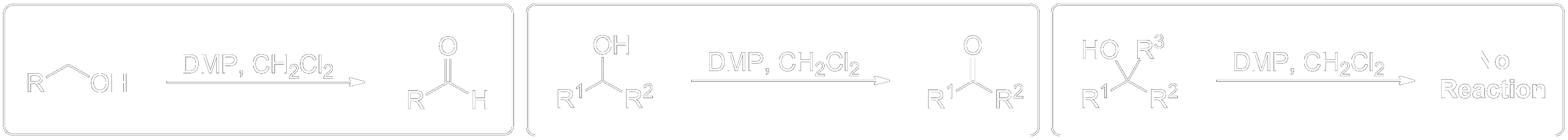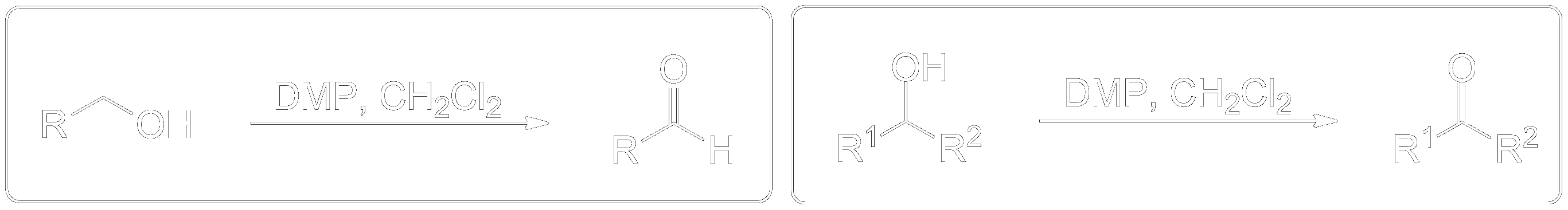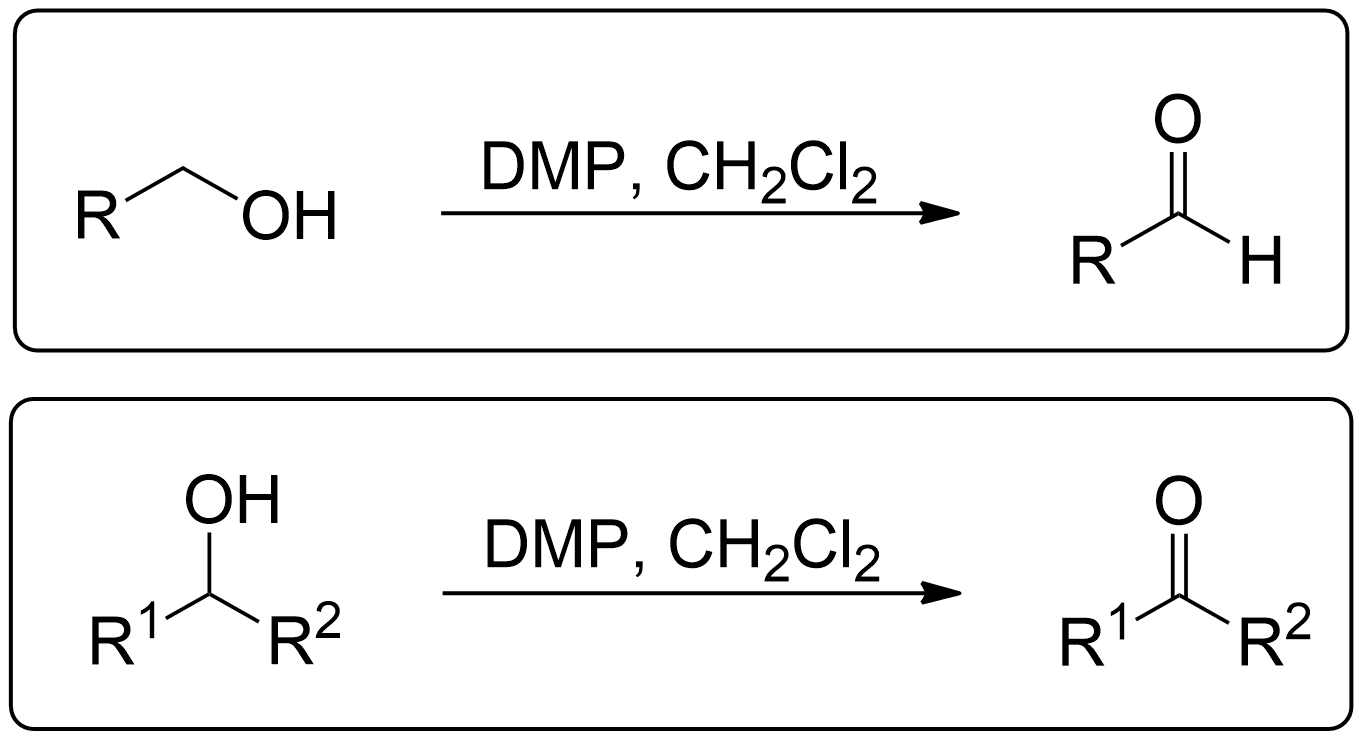
 The Dess-Martin oxidation is a method used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones using the Dess-Martin periodinane (DMP). 1
The Dess-Martin oxidation is a method used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones using the Dess-Martin periodinane (DMP). 1
Finding the Product for a 1° Alcohol
This section is a brief overview on how to find the product for a 1° Alcohol (Primary) using a example from a real scientific research paper.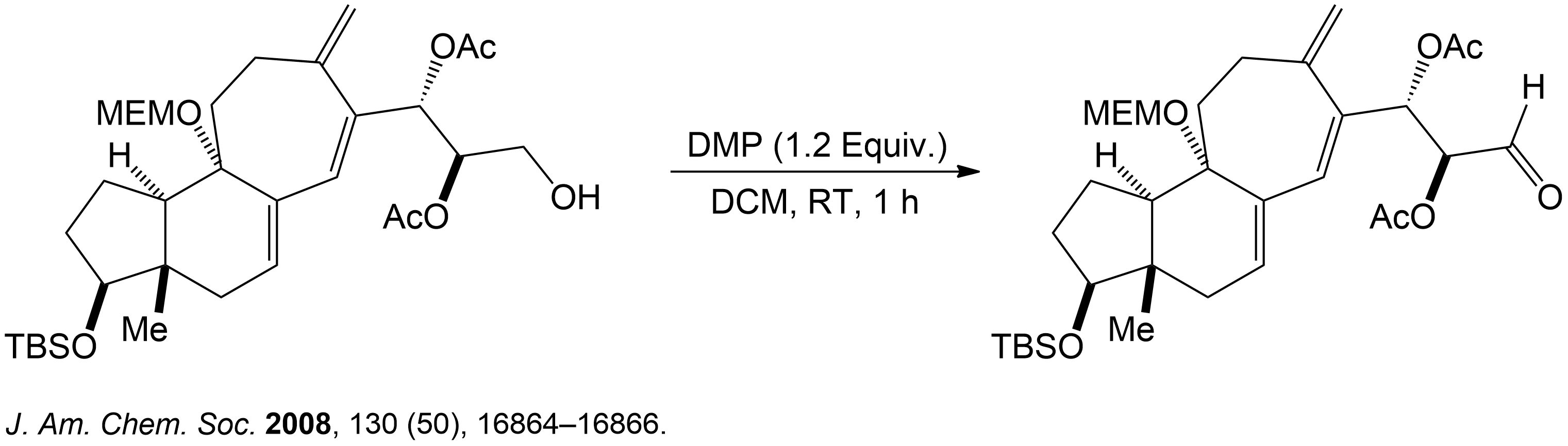
Propose a Mechanism.
Where did this Reaction come from?
Where did this Reaction come from?
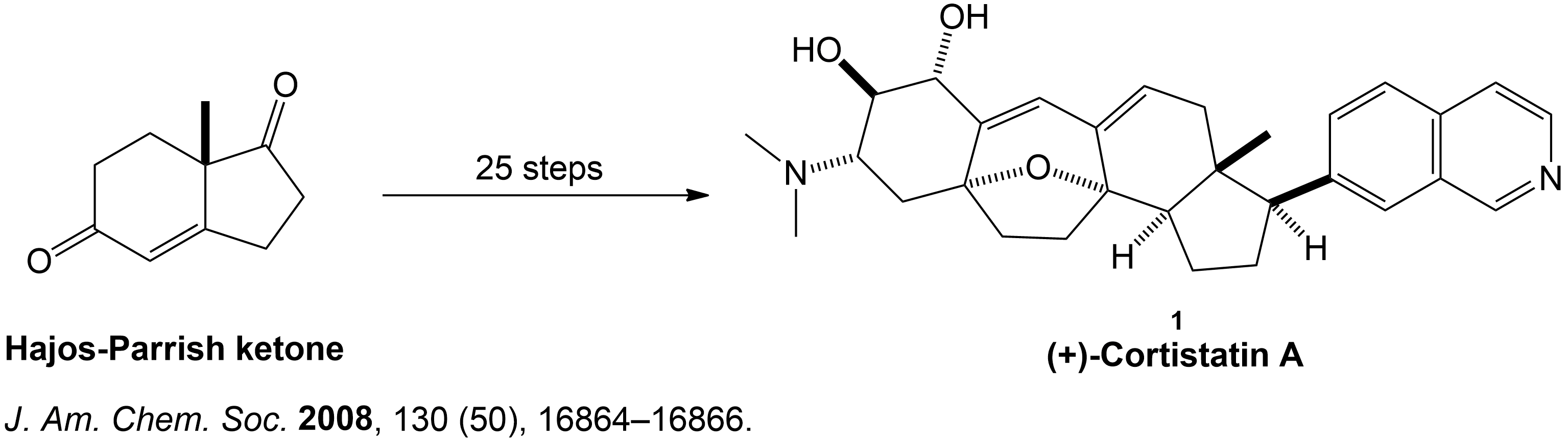
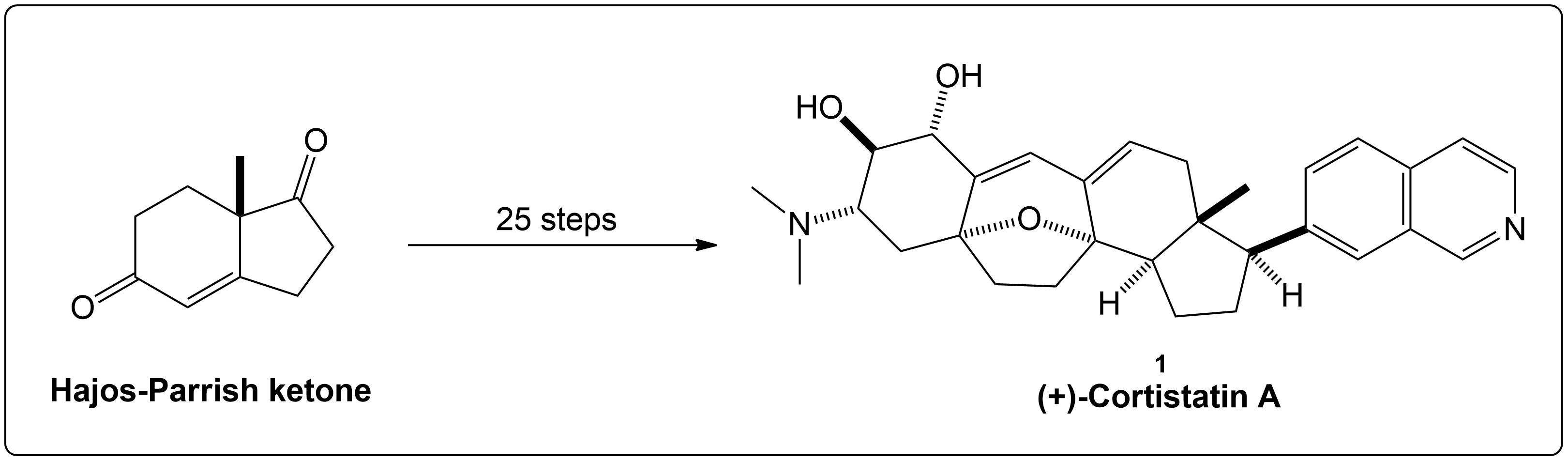
Condensed Synthesis Overview of (+)-Cortistatin A from Hajos-Parrish Ketone.
The overall synthesis of (+)-Cortistatin A started with an enantiomerically pure Hajos-Parrish ketone undergoing key reactions such as diastereoselective hydrogenation, Rubottom oxidation, the Dess-Martin oxidation and other types of reactions to eventually form the target compound (+)-Cortistatin A. This occurs over a 26 step synthesis pathway.
How is Dess-Martin Oxidation used?
How is Dess-Martin Oxidation used?
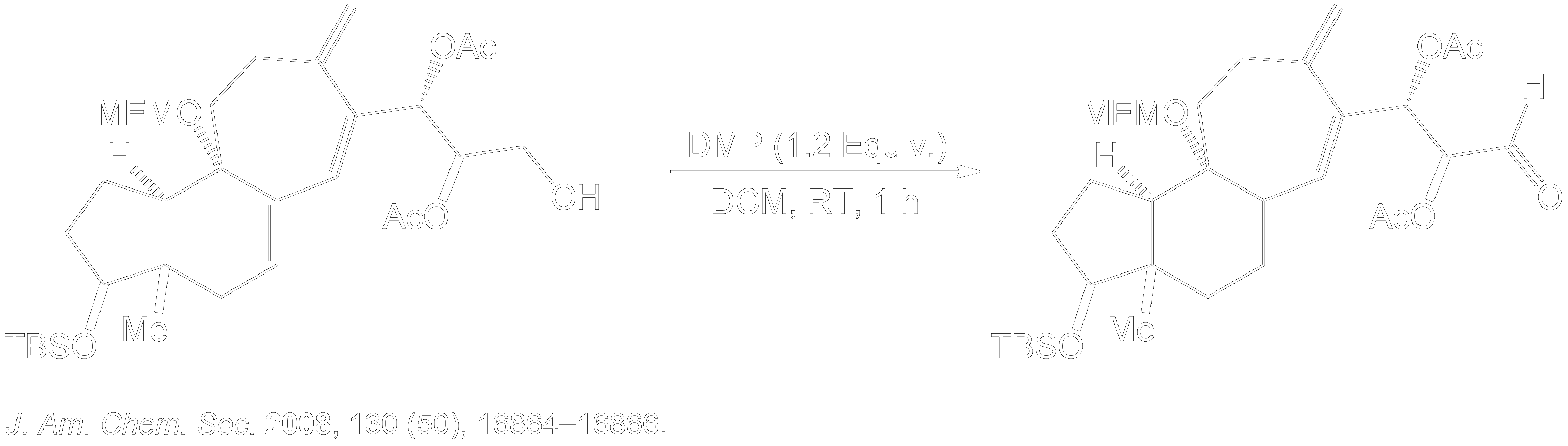
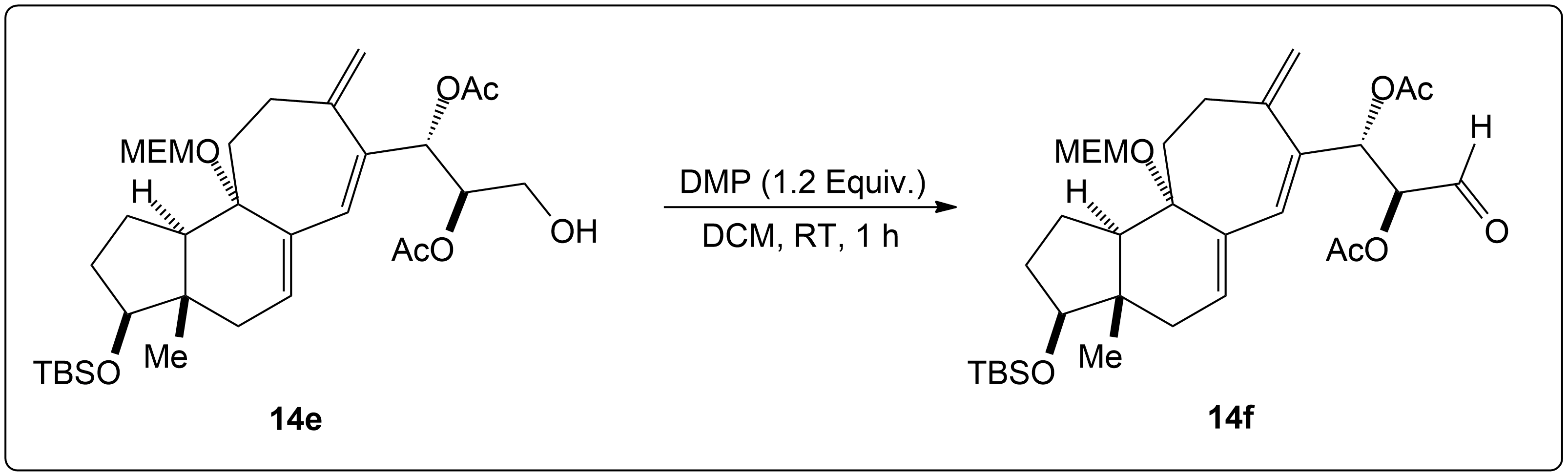
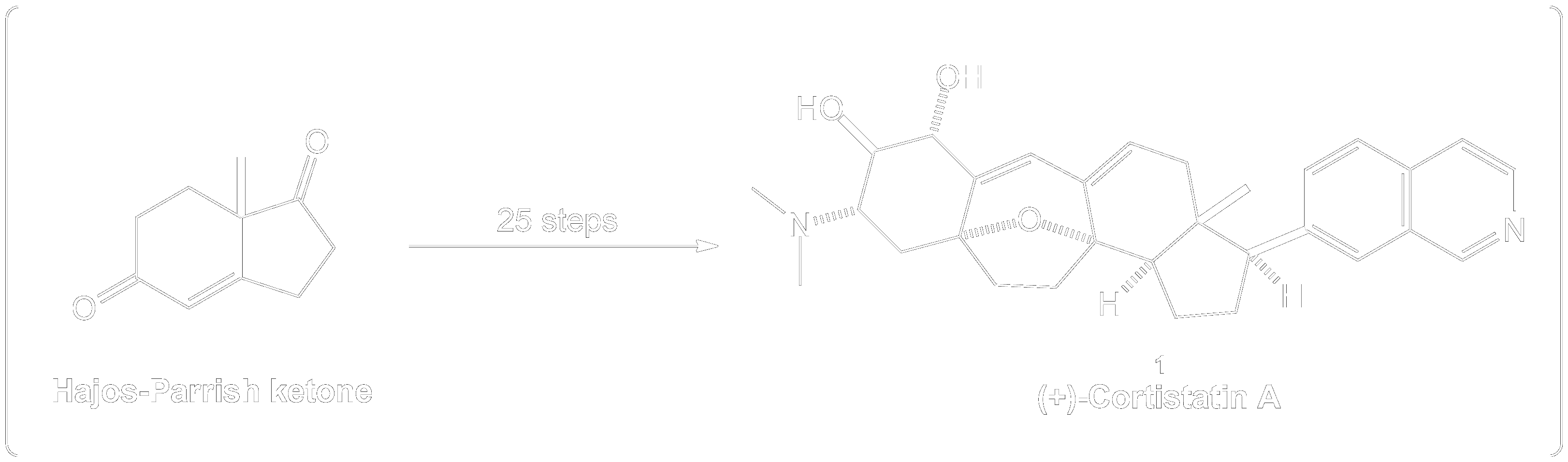
Dess-Martin Oxidation Usage in Intermediate Formation.
This reaction showing the Dess-Martin oxidation of this alcohol intermediate was part of the overall synthesis pathway of the formation of (+)-Cortistatin A. (+)-Cortistatin A is a potent inhibitor of endothelial cell proliferation. This compound was synthesized through the conversion of an enantiomerically pure Hajos-Parrish ketone to a known enone and then to a silyloxydiene intermediate. 2
Identify the Right Reagents
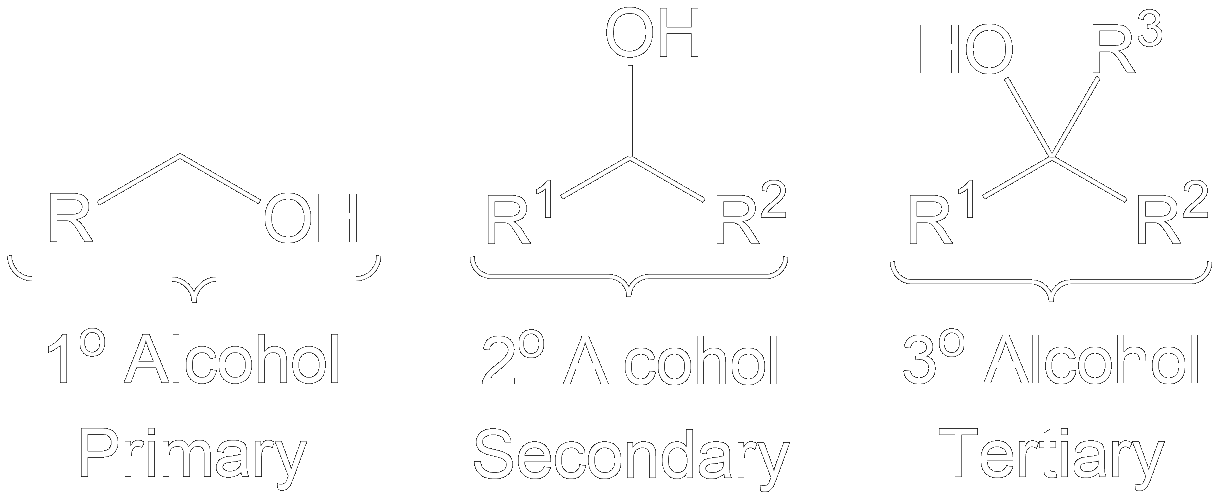
Identify the Key Features of the Compound
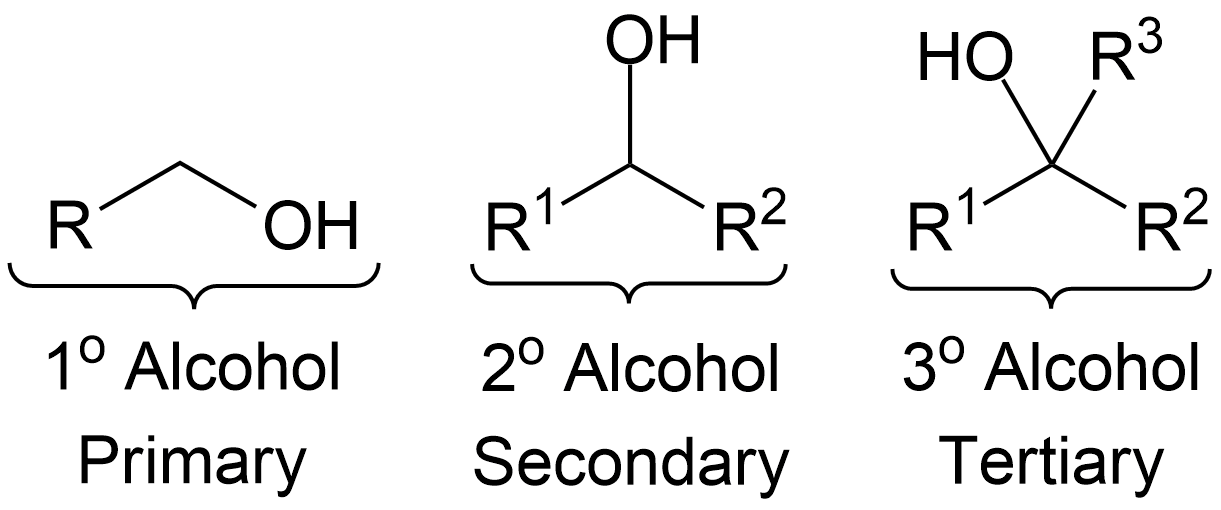
- Primary
- Secondary
- Tertiary
- Primary alcohols can go through Dess-Martin Oxidation to become an Aldehyde.
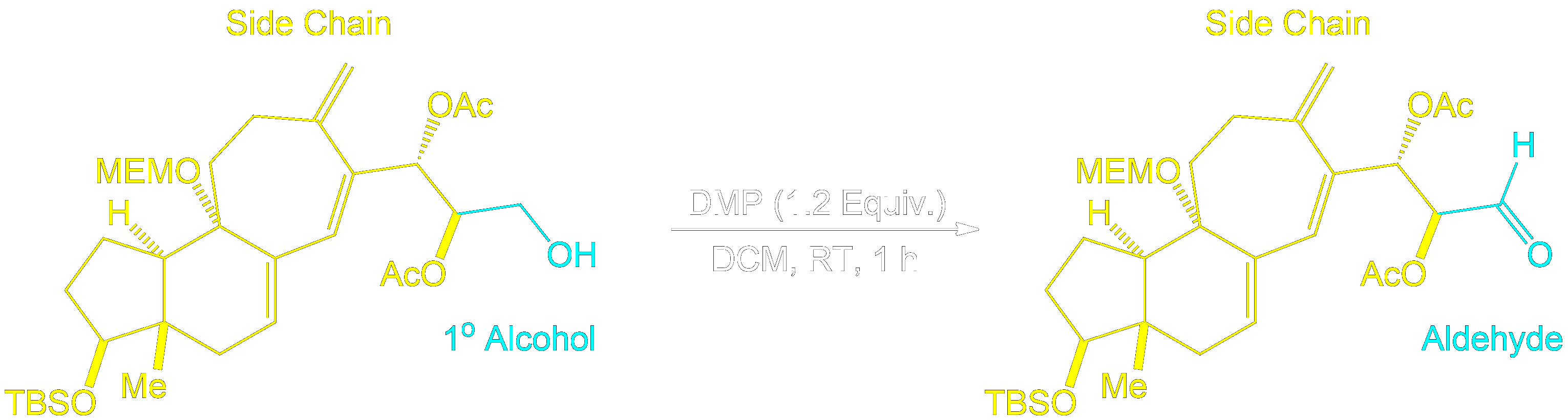
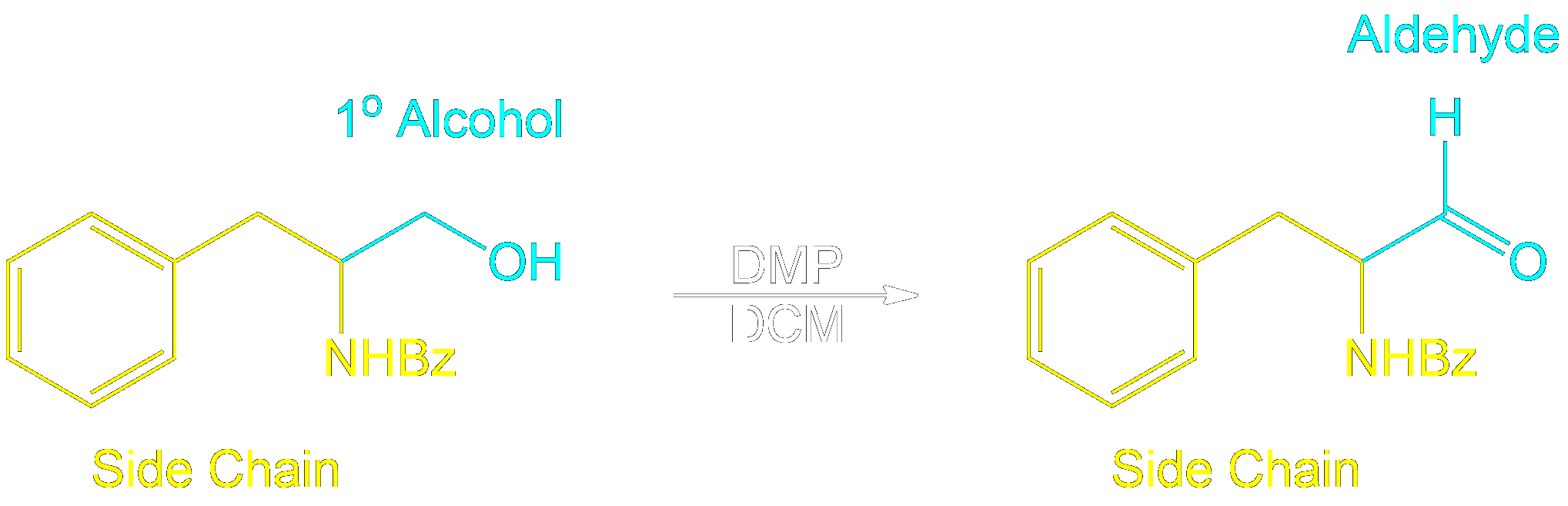
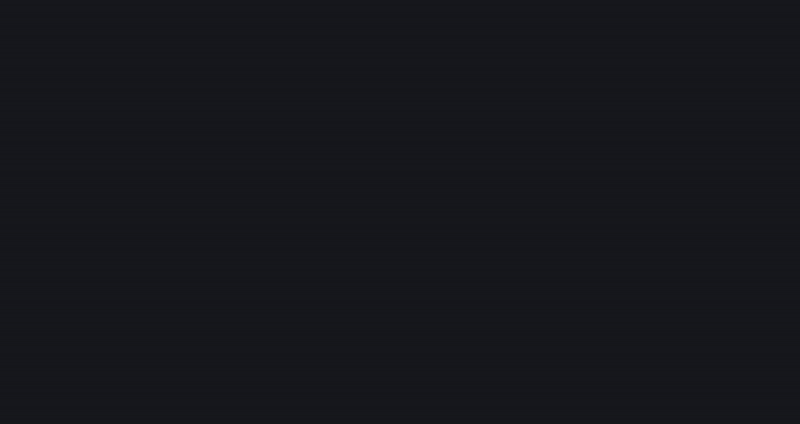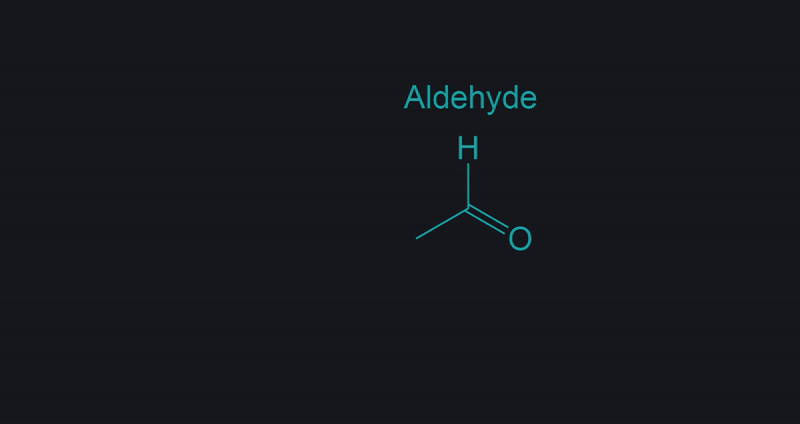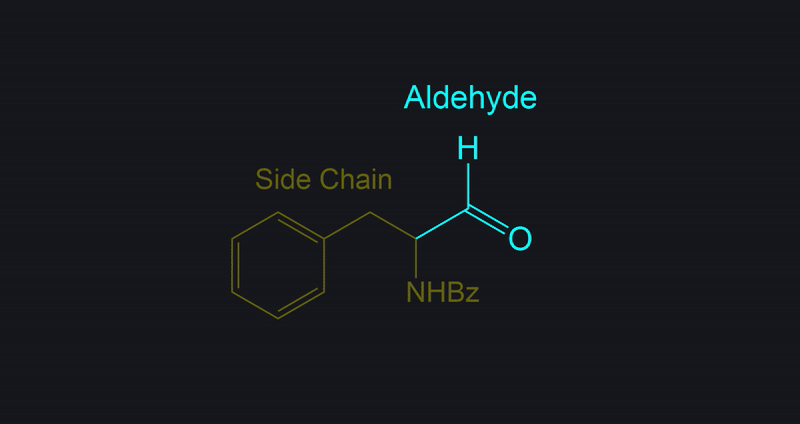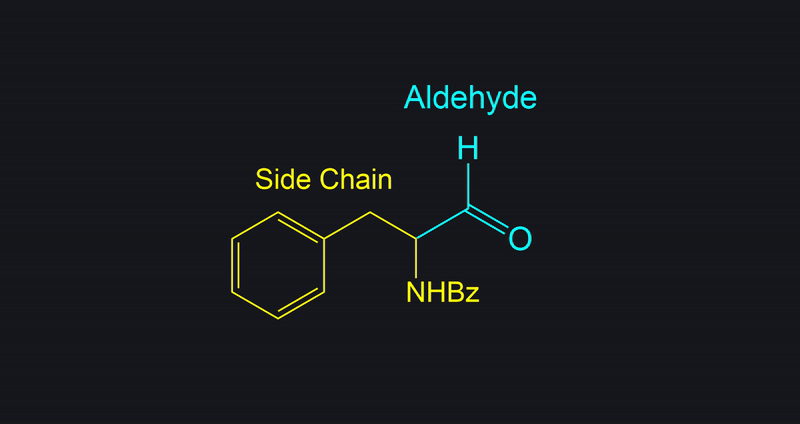
Identifying Side Chains and Alcohol Conversion
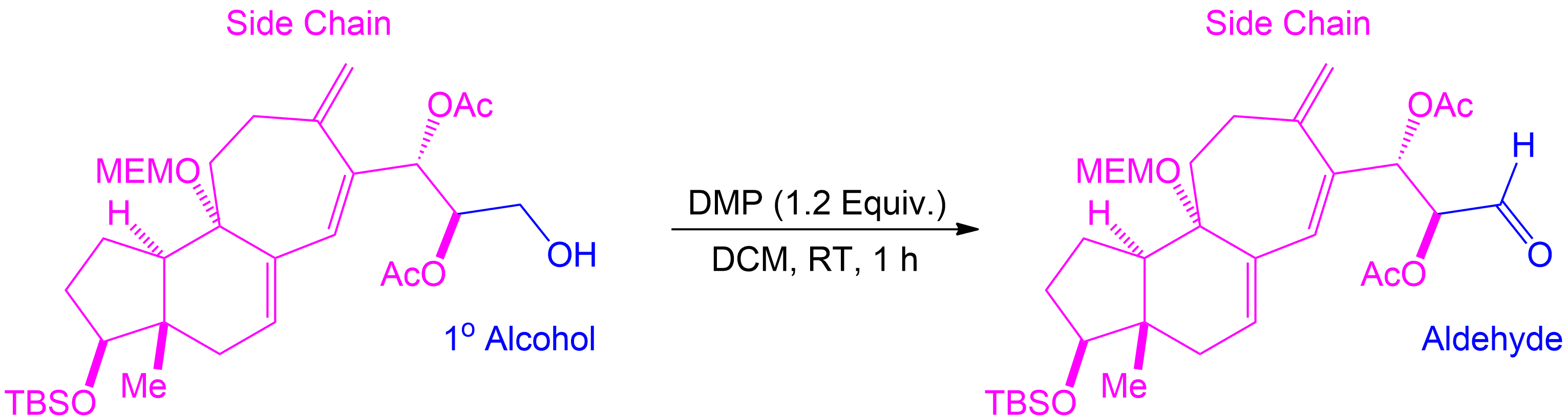
Tracking Side Chains and Alcohol Conversion.
In Dess-Martin oxidation of primary alcohols, the process involves assigning one side chain (R) to understand the reaction better.
- Assign the Side Chain (R): Identify the non-alcohol part of the molecule and assign it as the placeholder ‘R’ or side chain.
- Understand Its Role: This placeholder helps track the unchanged part of the molecule, aiding in visualizing the structure before and after the reaction.
- Focus on the Reaction Center: The primary alcohol is selectively oxidized to form an aldehyde. The placeholder shows how the structure is altered.
- Reassign the Side Chain: After the reaction, reattach the placeholder R to the new aldehyde, demonstrating the unchanged nature of the side chain.
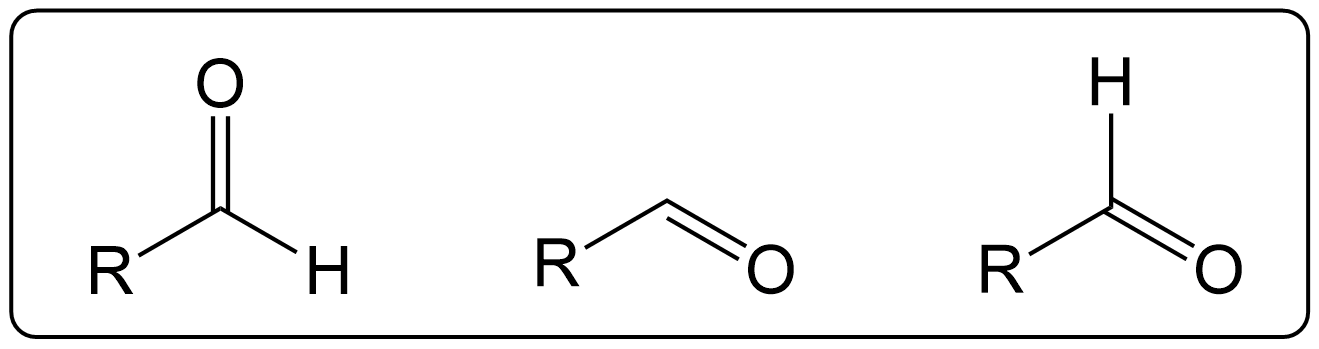
Variations on how Aldehydes may appear.
They may be differently presented in different questions as shown in the image, however they are the same structure.
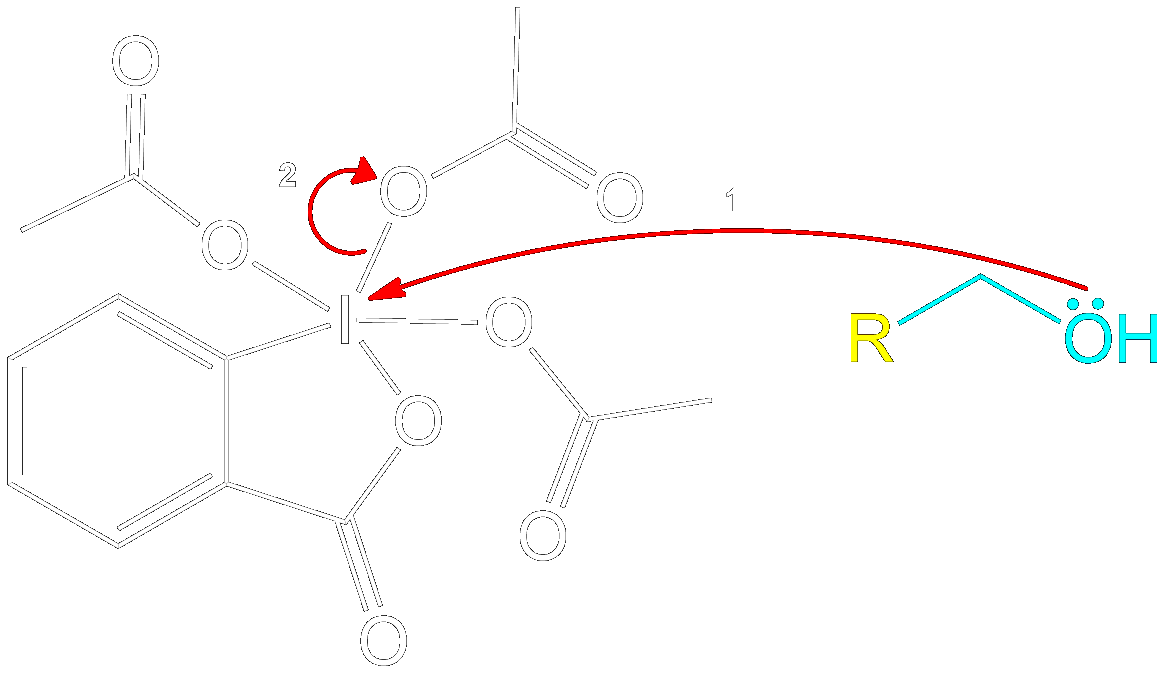
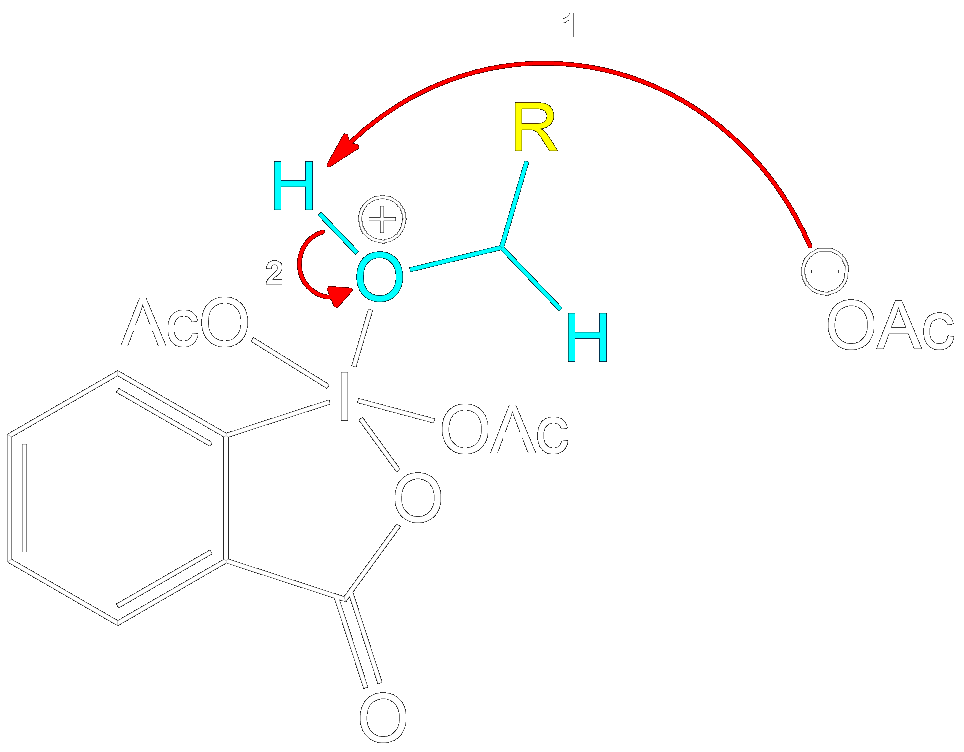
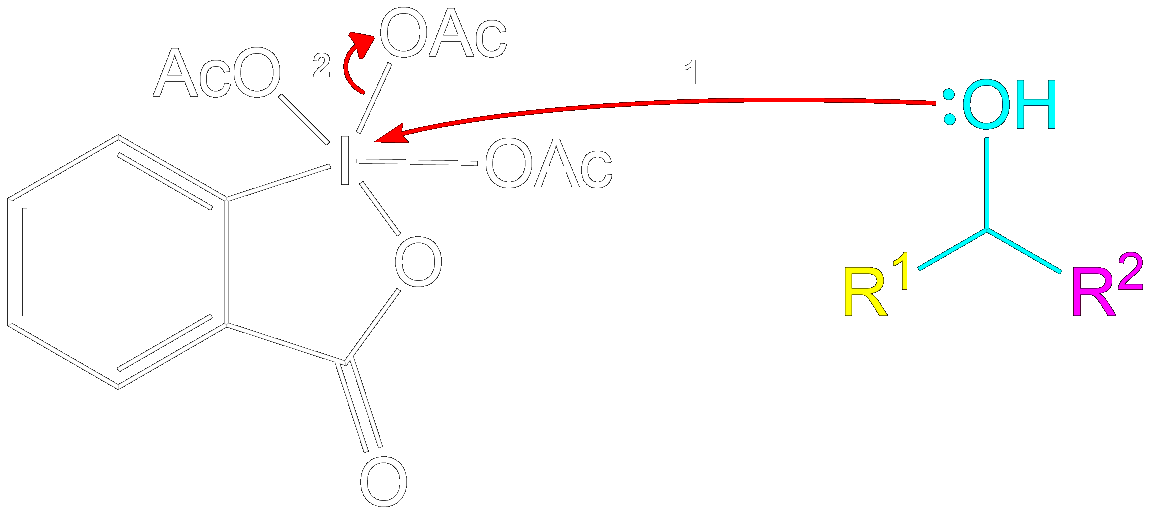
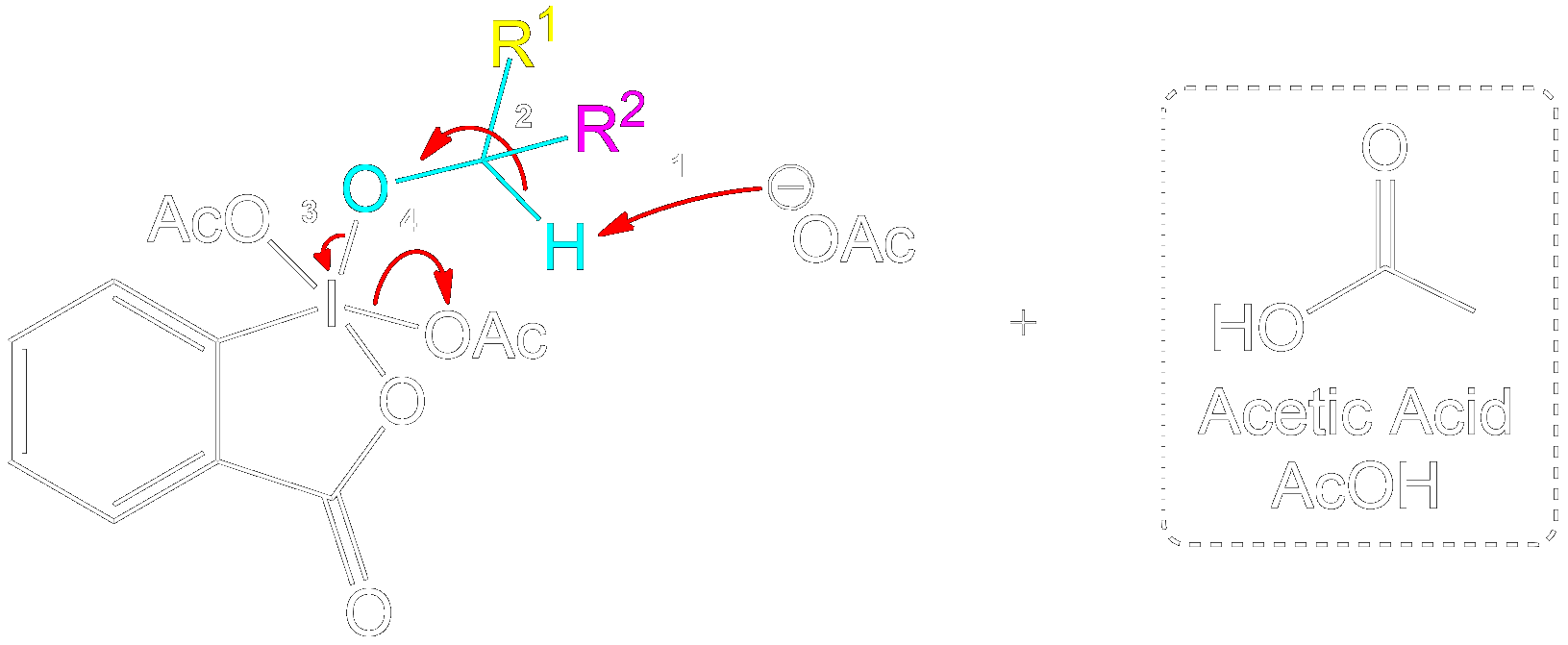
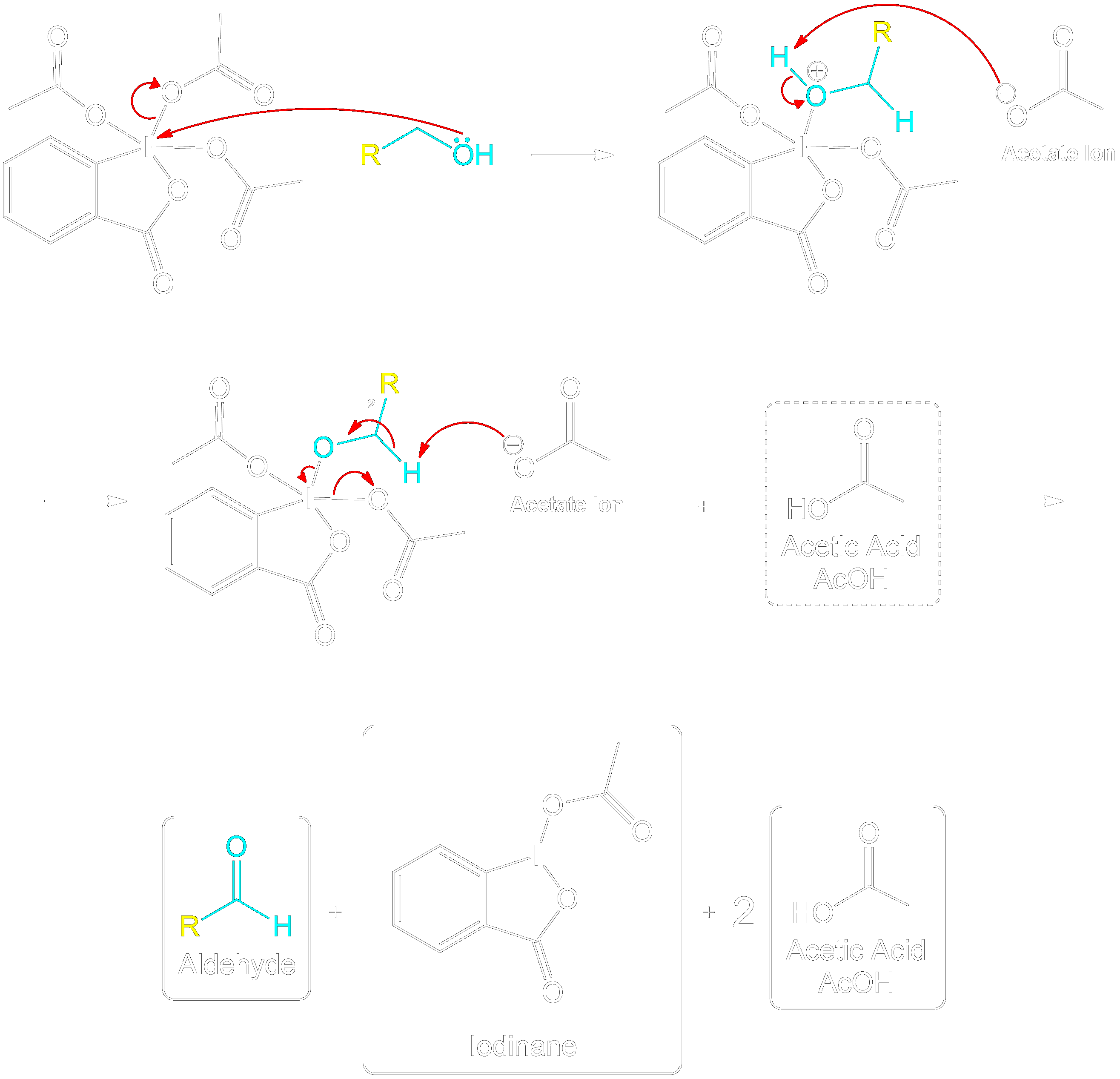
Mechanism for 1° Alcohol
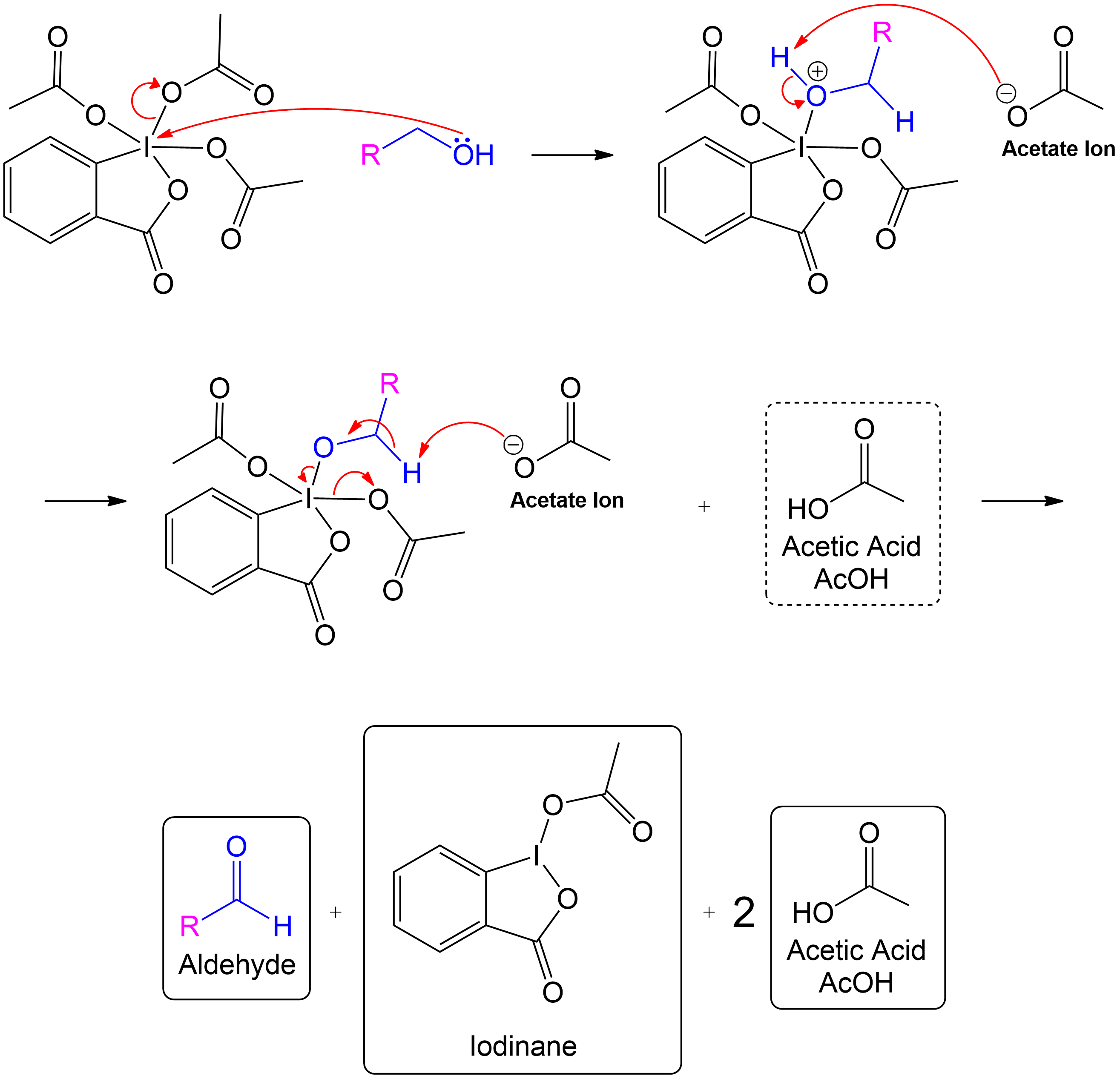
This section is a brief overview on how to perform the mechanism for a 1° Alcohol (Primary) using the example from above.Reactive Intermediate Formation
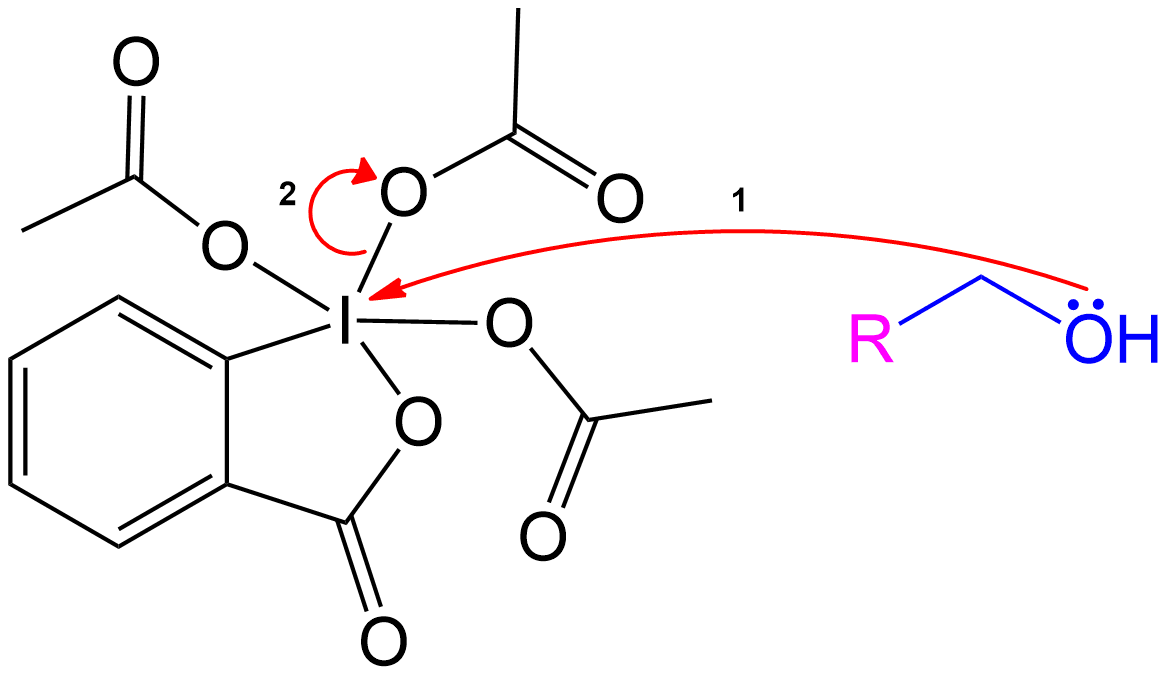
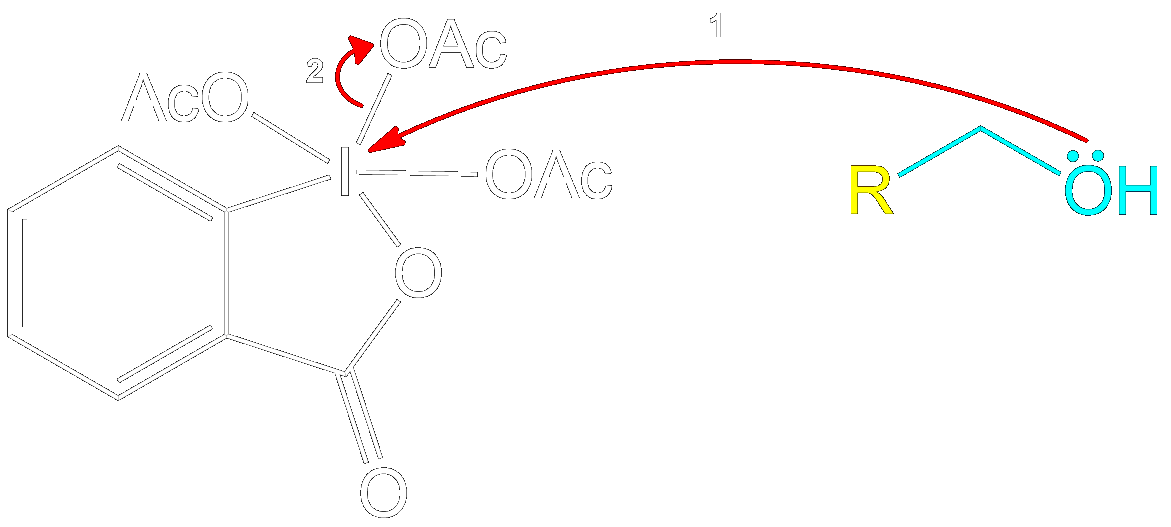
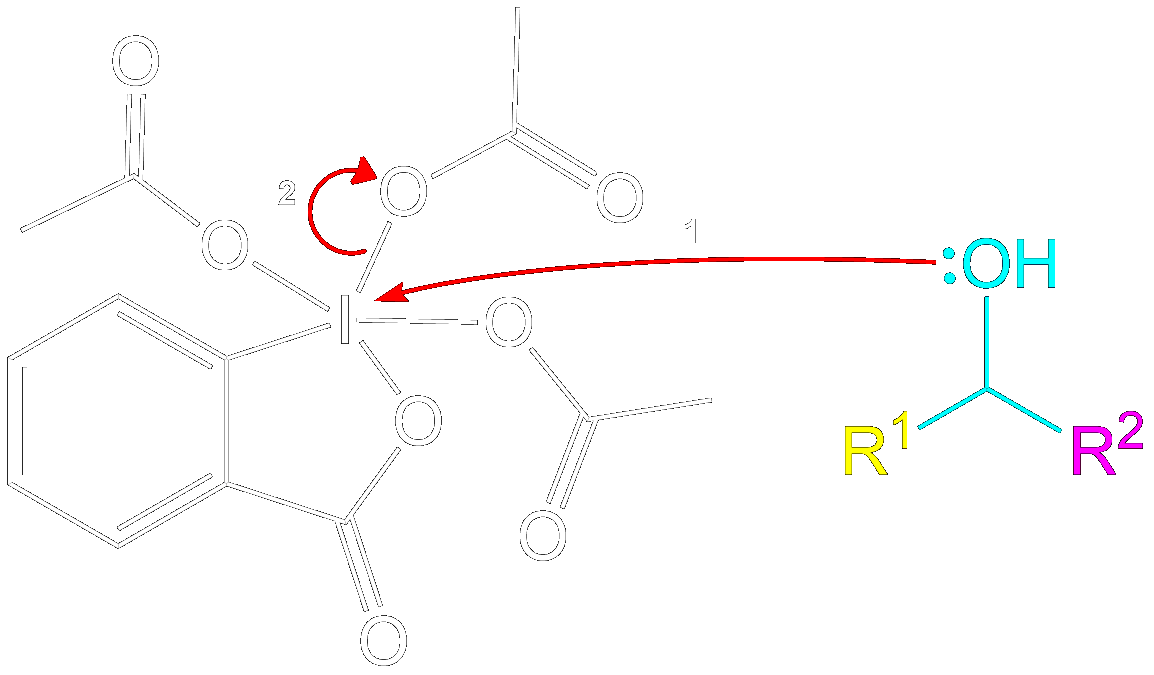
Formation of Reactive Intermediate.
Alcohol group (-OH) performs a nucleophilic attack on the iodine center of the DMP molecule, forming a complex with the iodine. Acetoxy group is expelled and acts as a good leaving group that leaves with an extra electron pair.
See the Shortform Version of this Step
See the Shortform Version of this Step
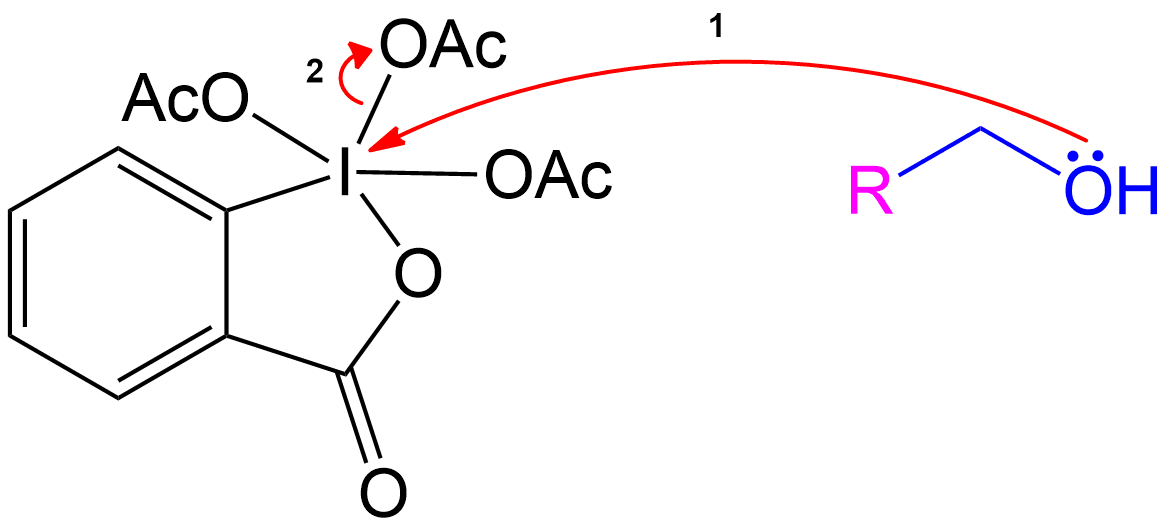
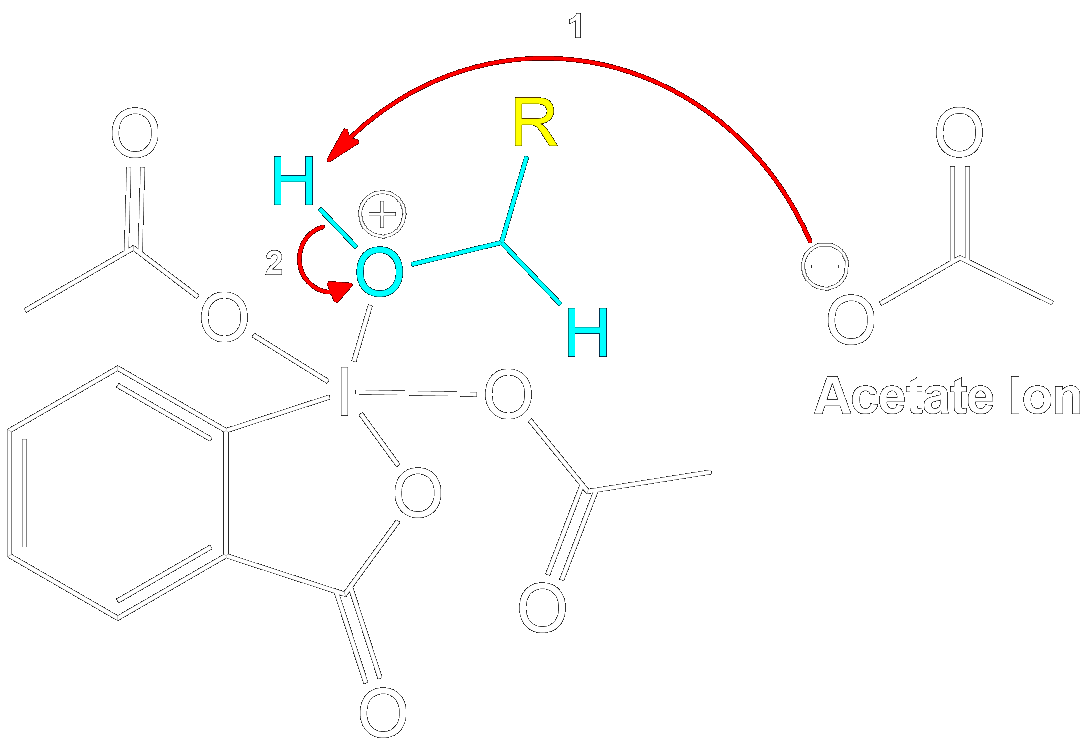
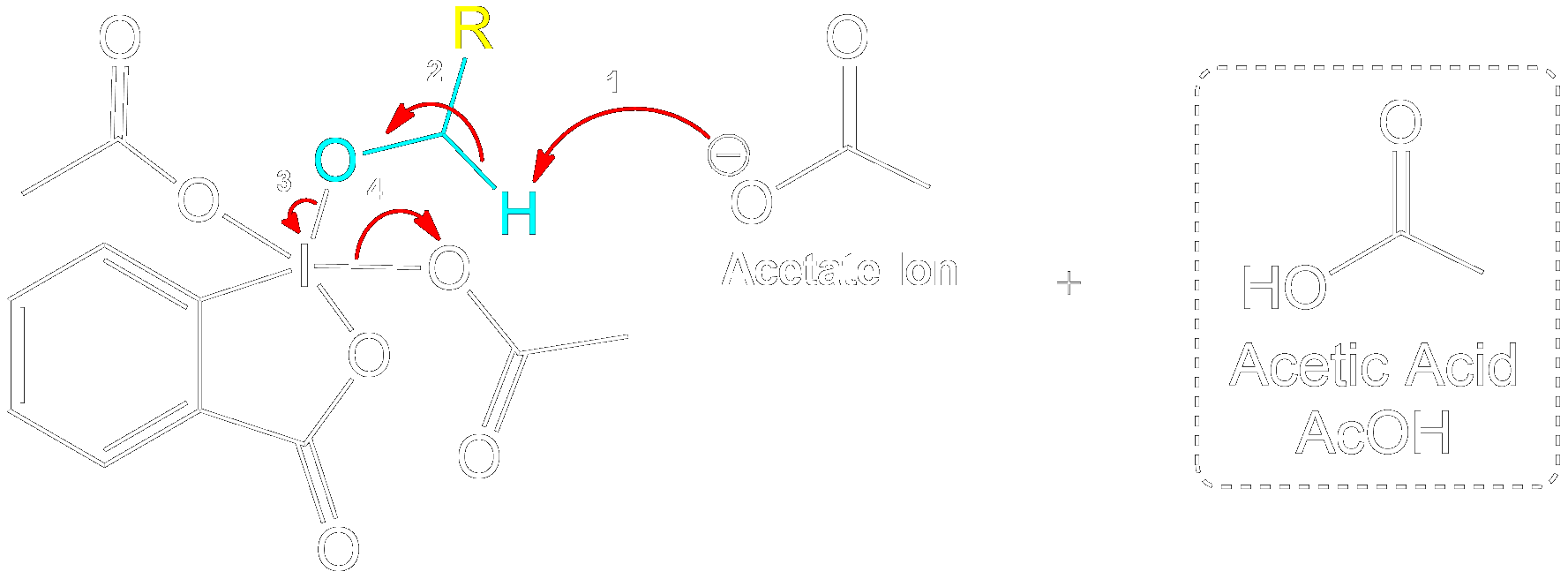
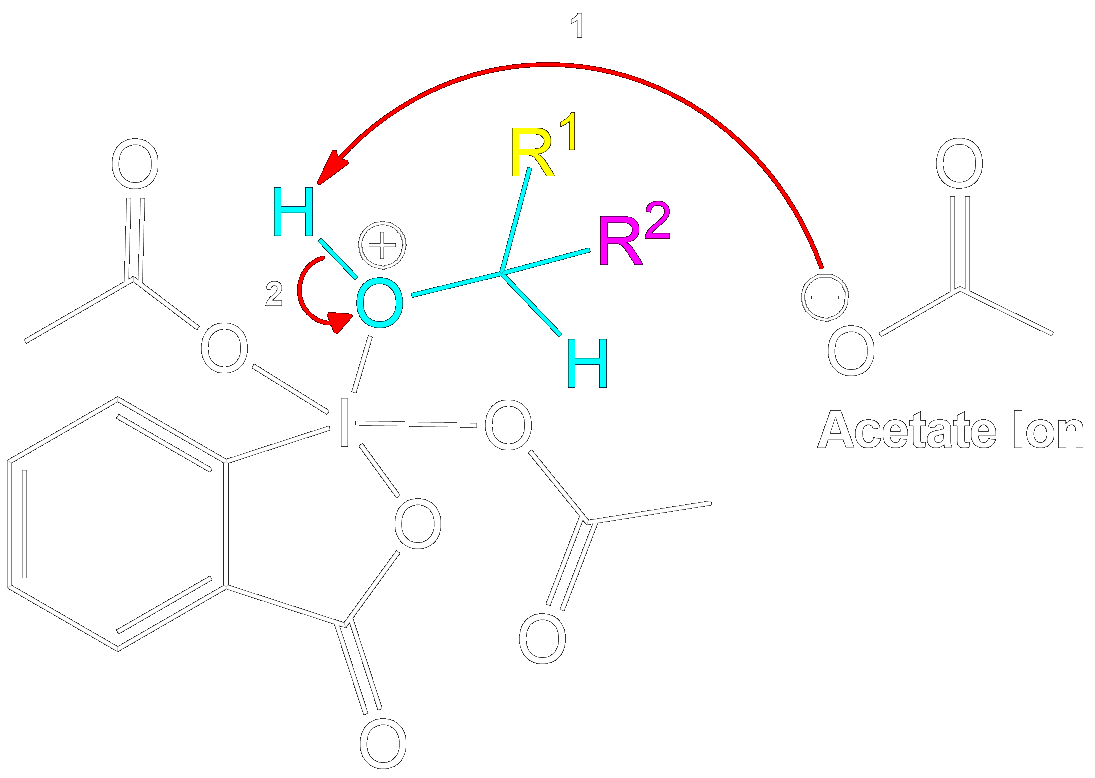
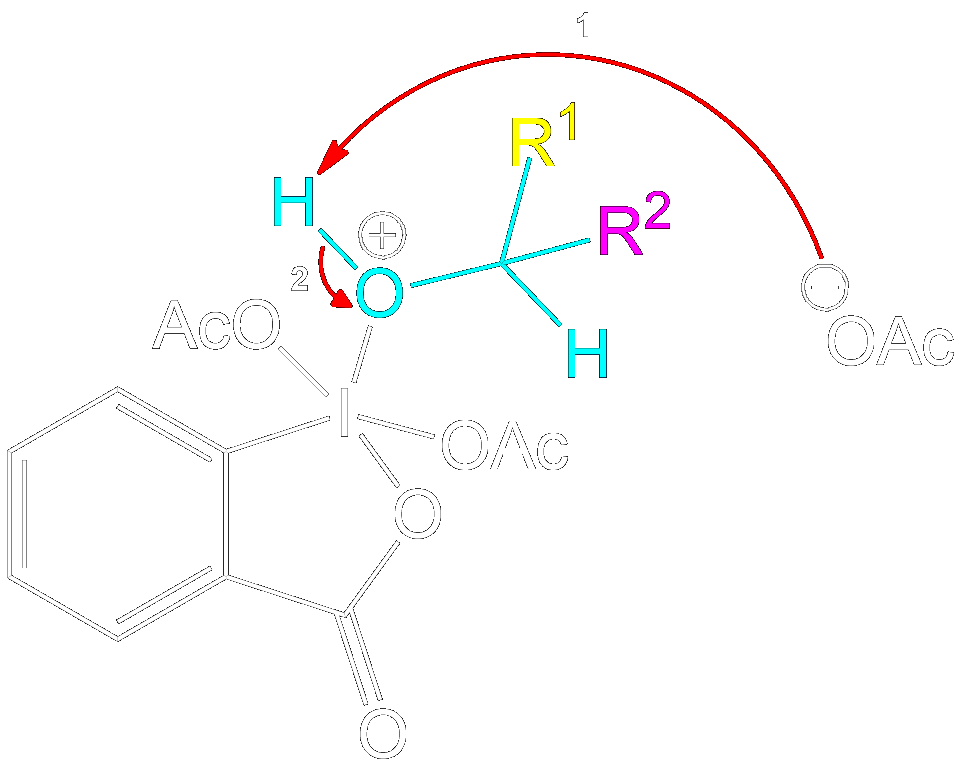
Stabilization with Acetate Ion
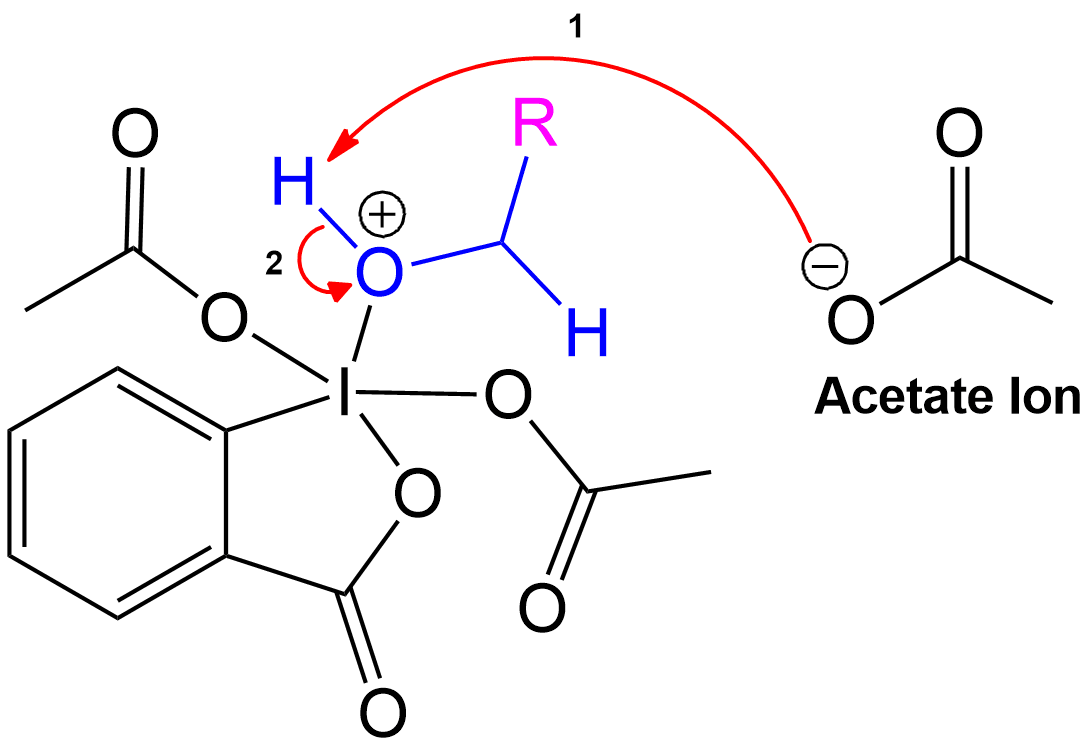
Charge Stabilization using a Acetate Ion.
The newly formed intermediate is stabilized by the previously expelled OAc (Acetoxy) group (now an acetate ion).
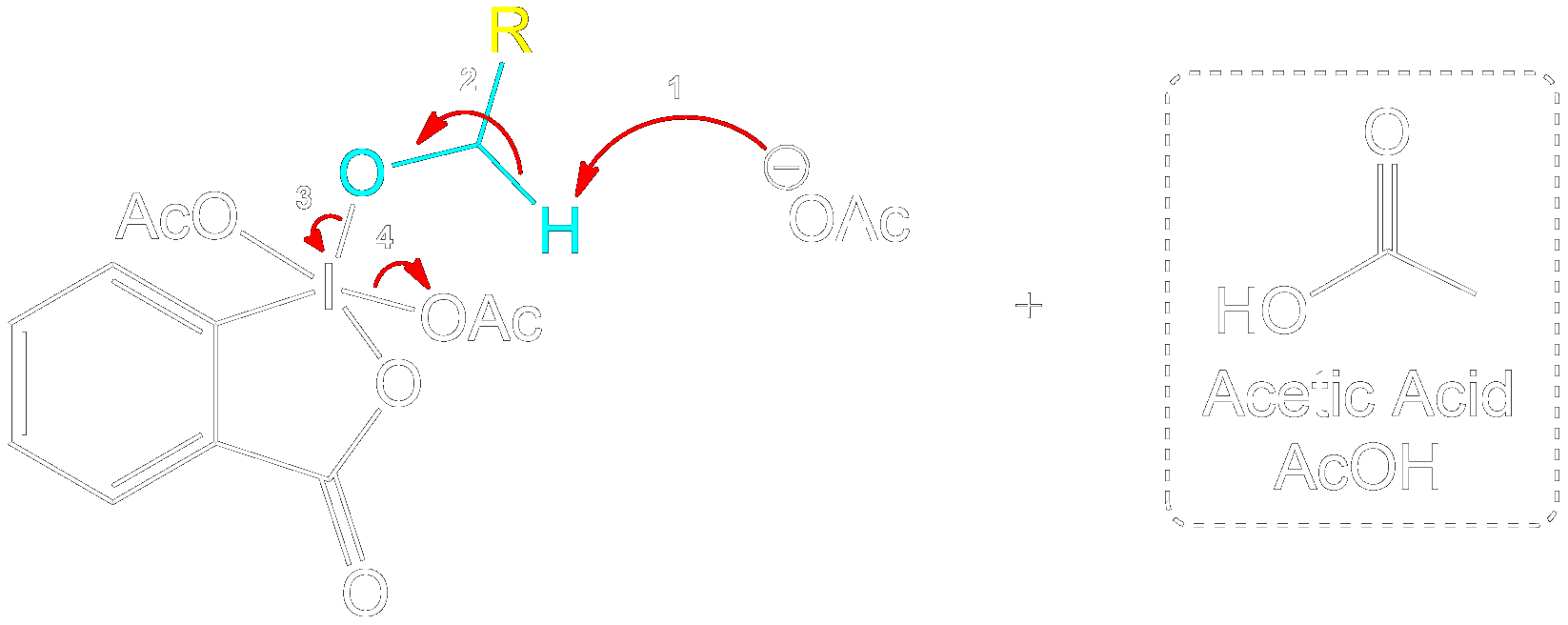
See the Shortform Version of this Step
See the Shortform Version of this Step
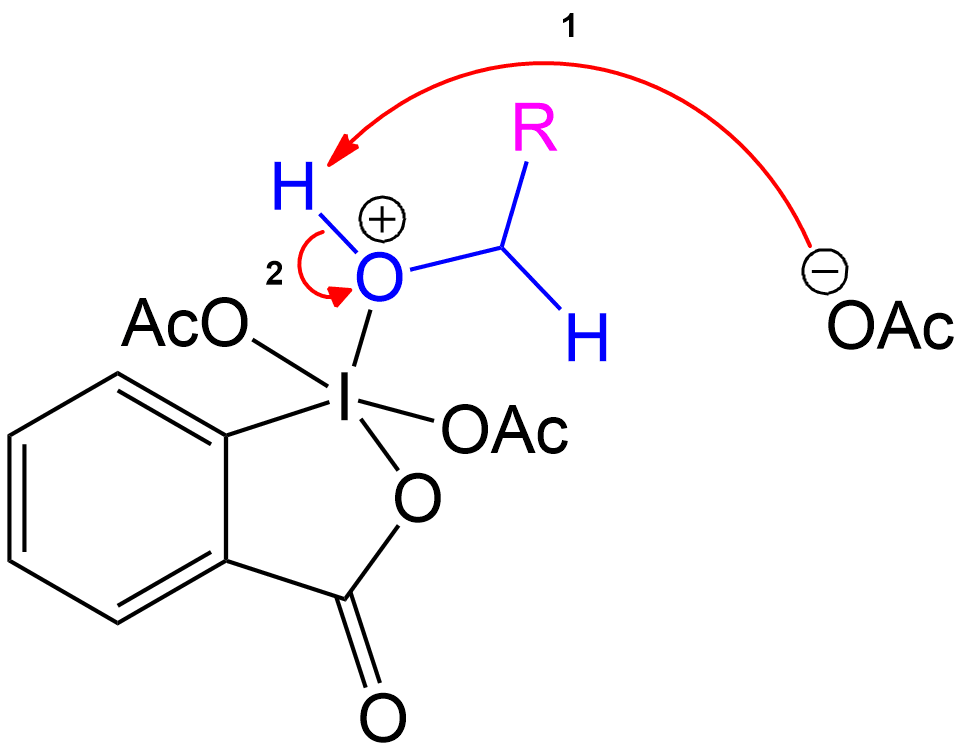
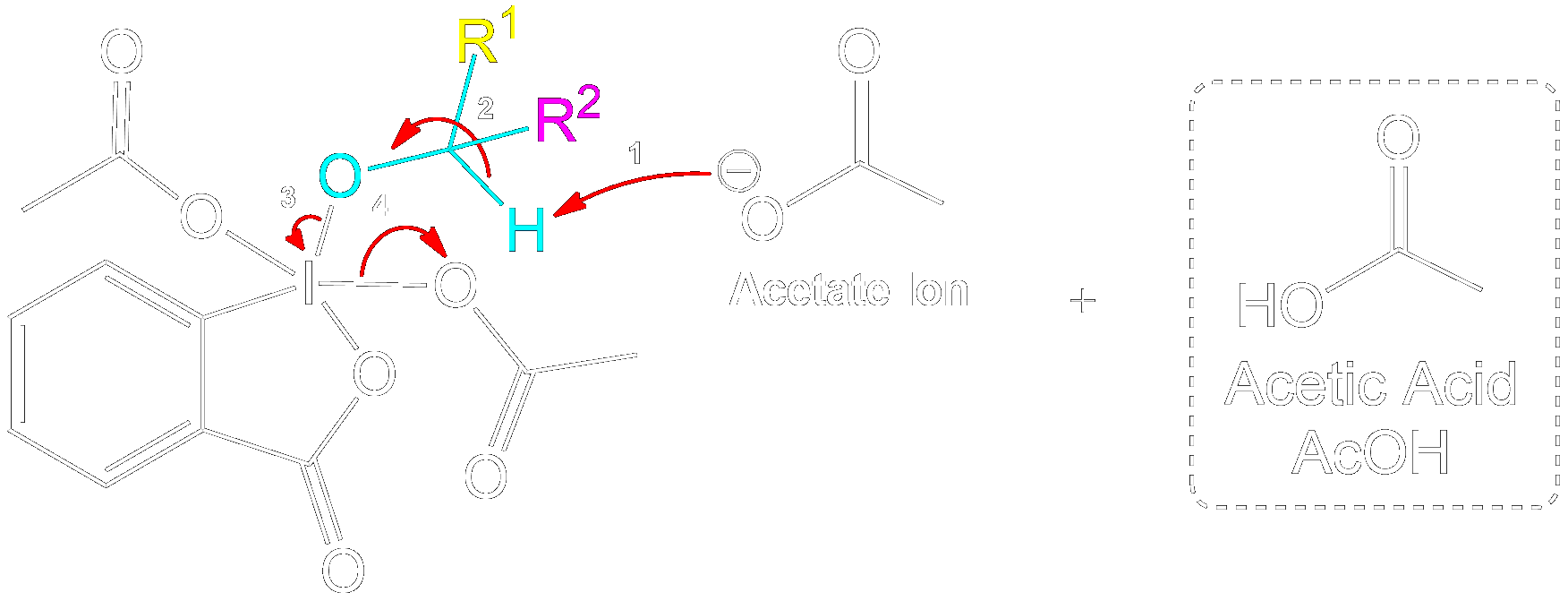
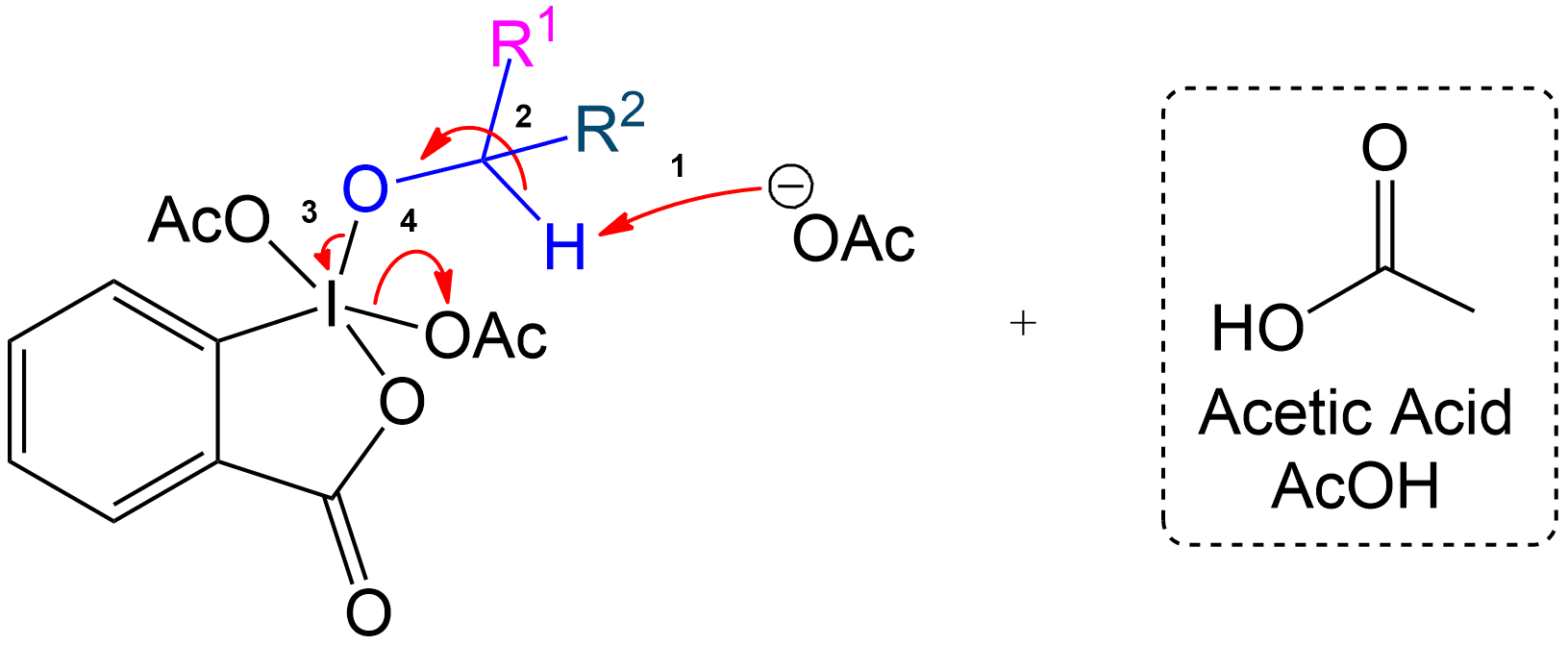
Product Formation and Acetic Acid Recovery
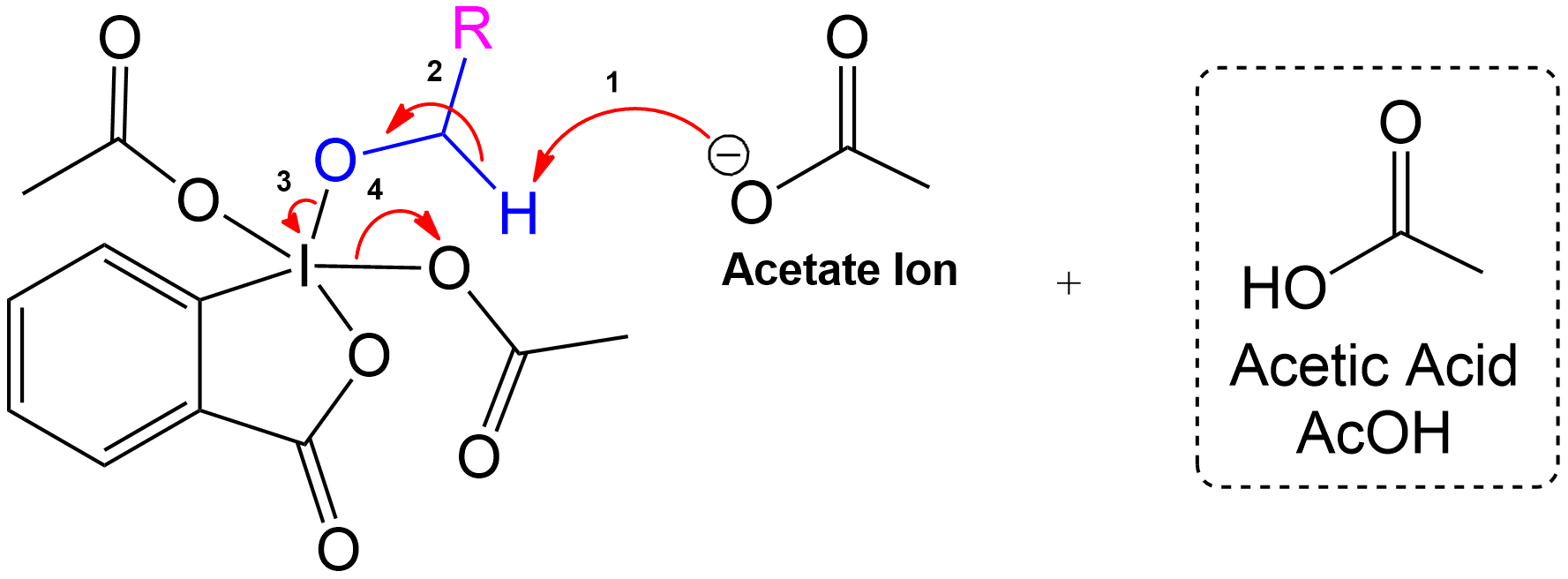
Acetic Acid Recovery and Product Formation.
Acetic acid is recovered, and the intermediate undergoes proton transfer initiated by another acetate ion to form the aldehyde product.
See the Shortform Version of this Step
See the Shortform Version of this Step
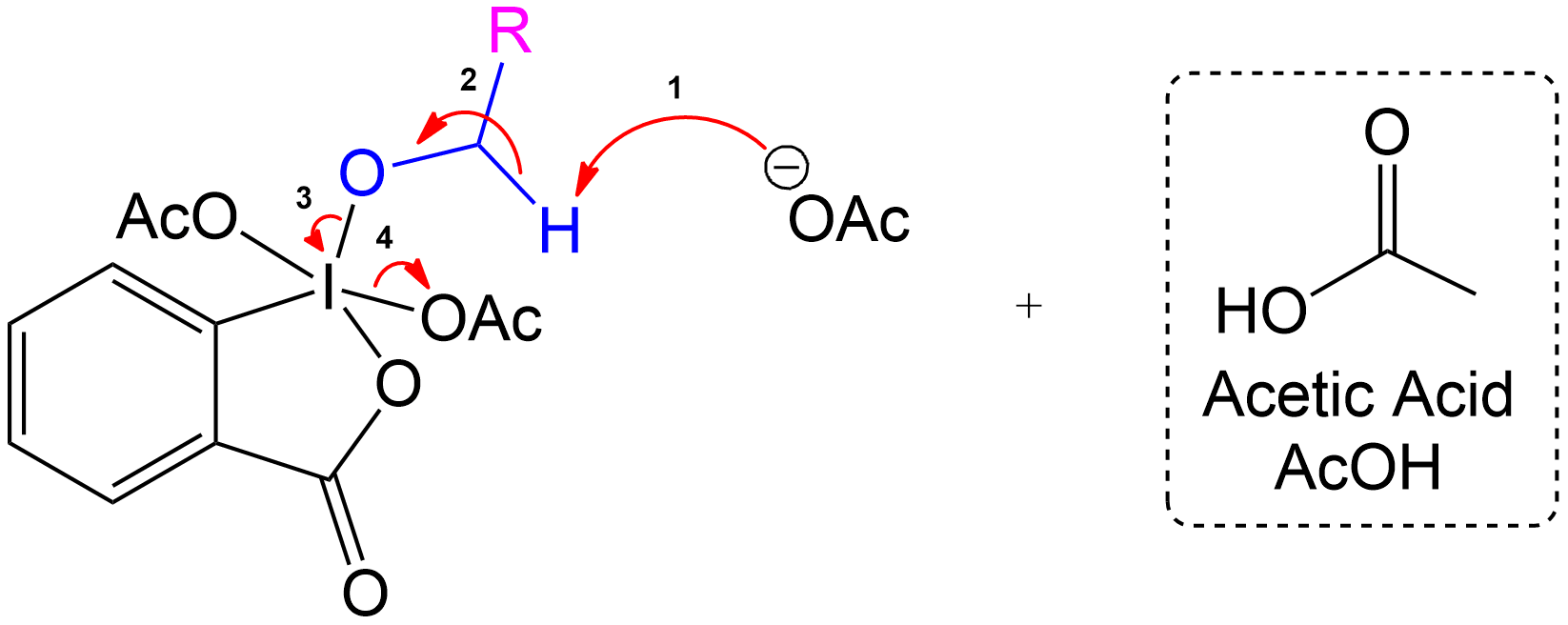
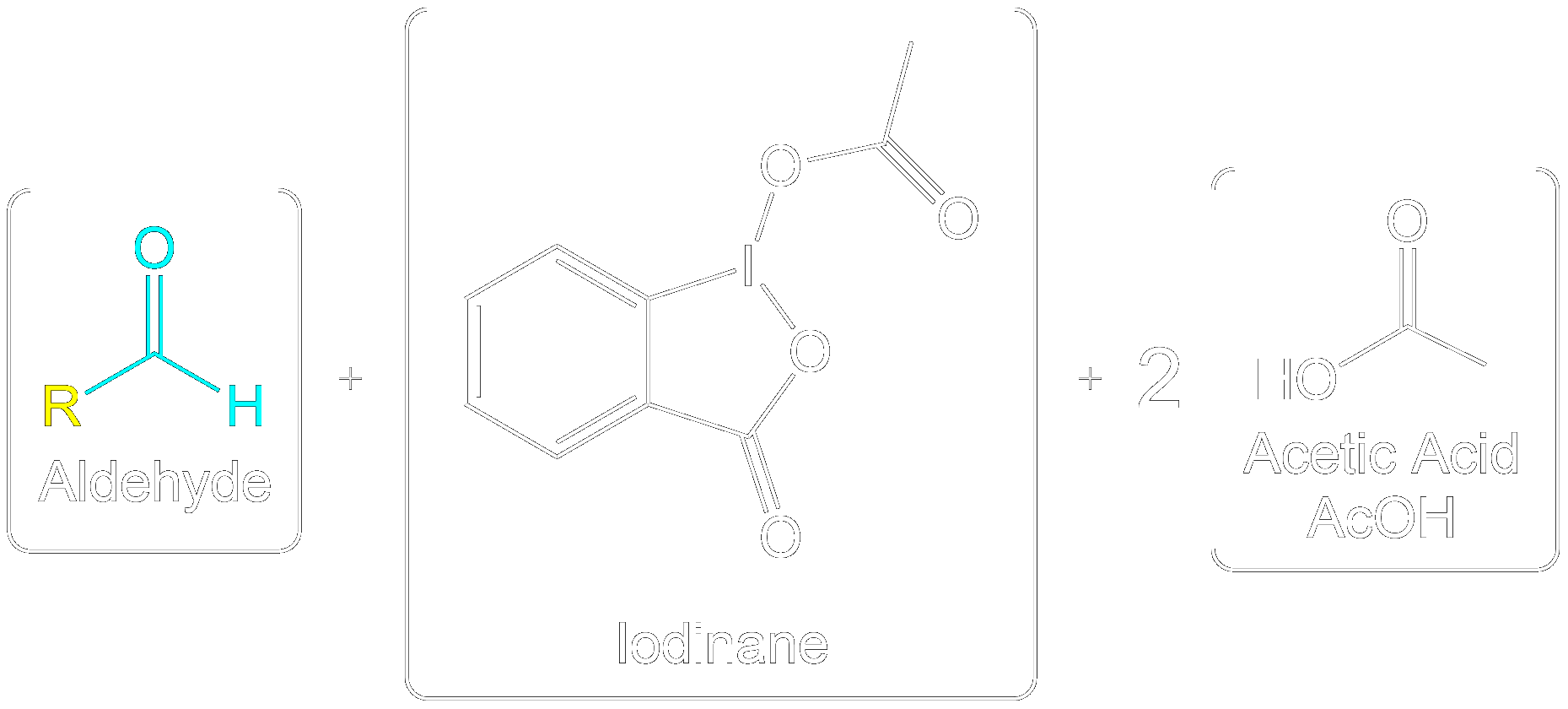
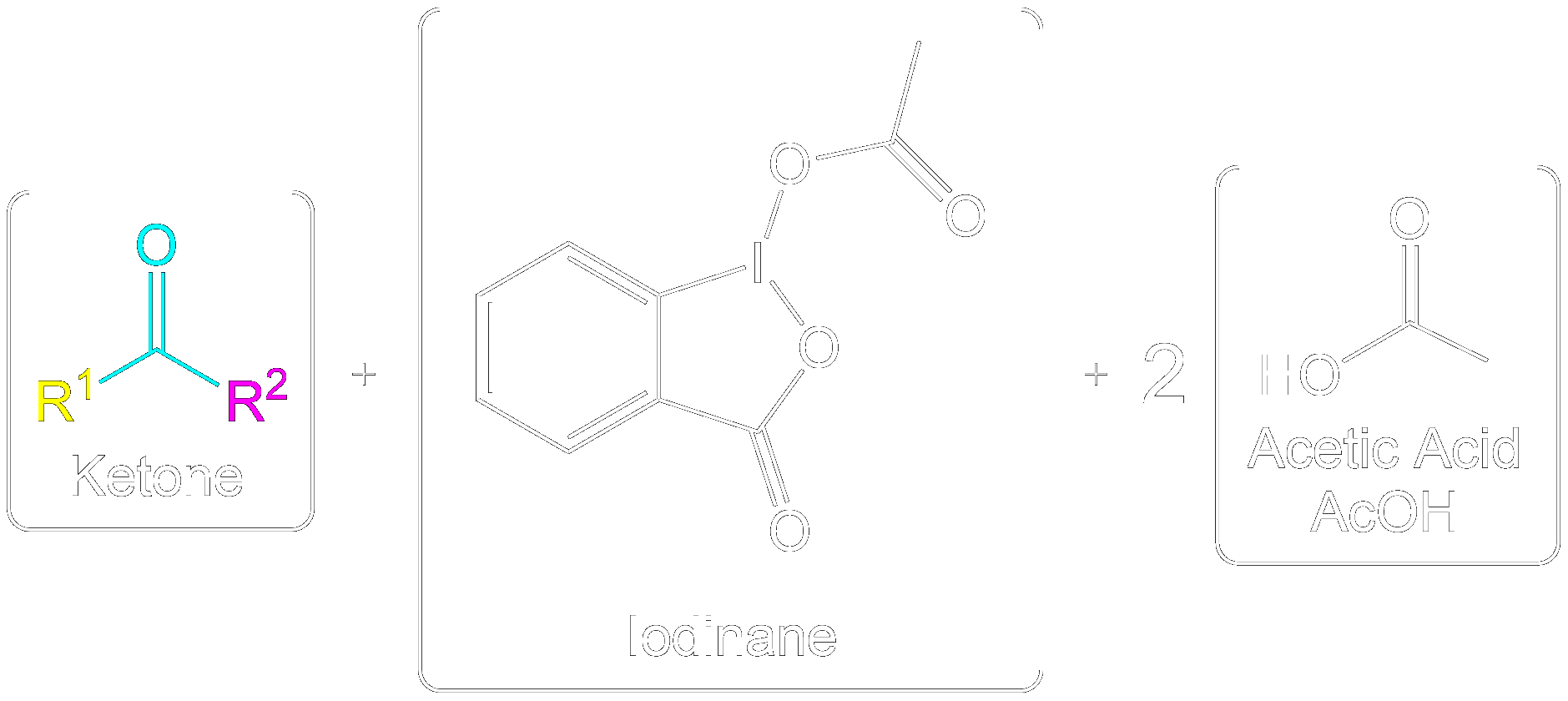
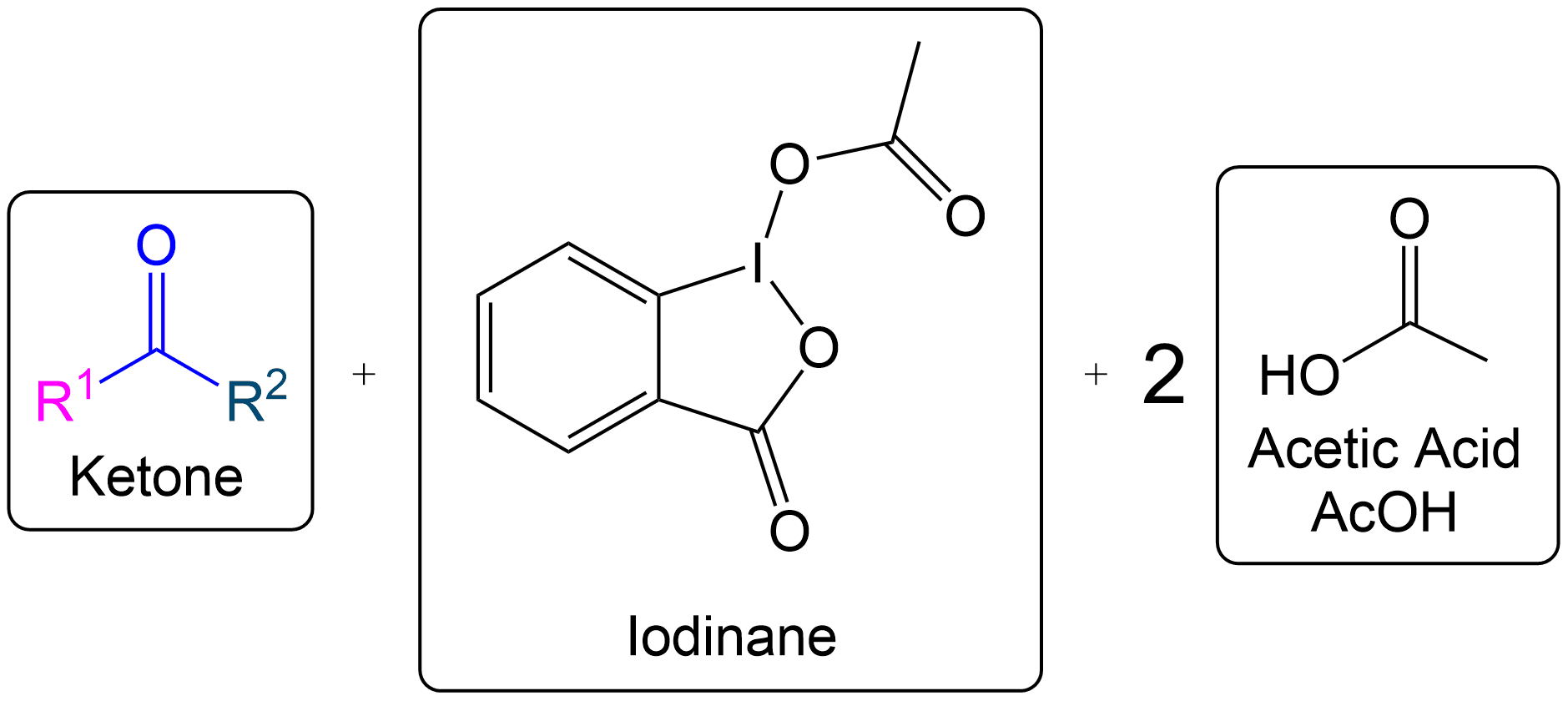
Overview of Final Products
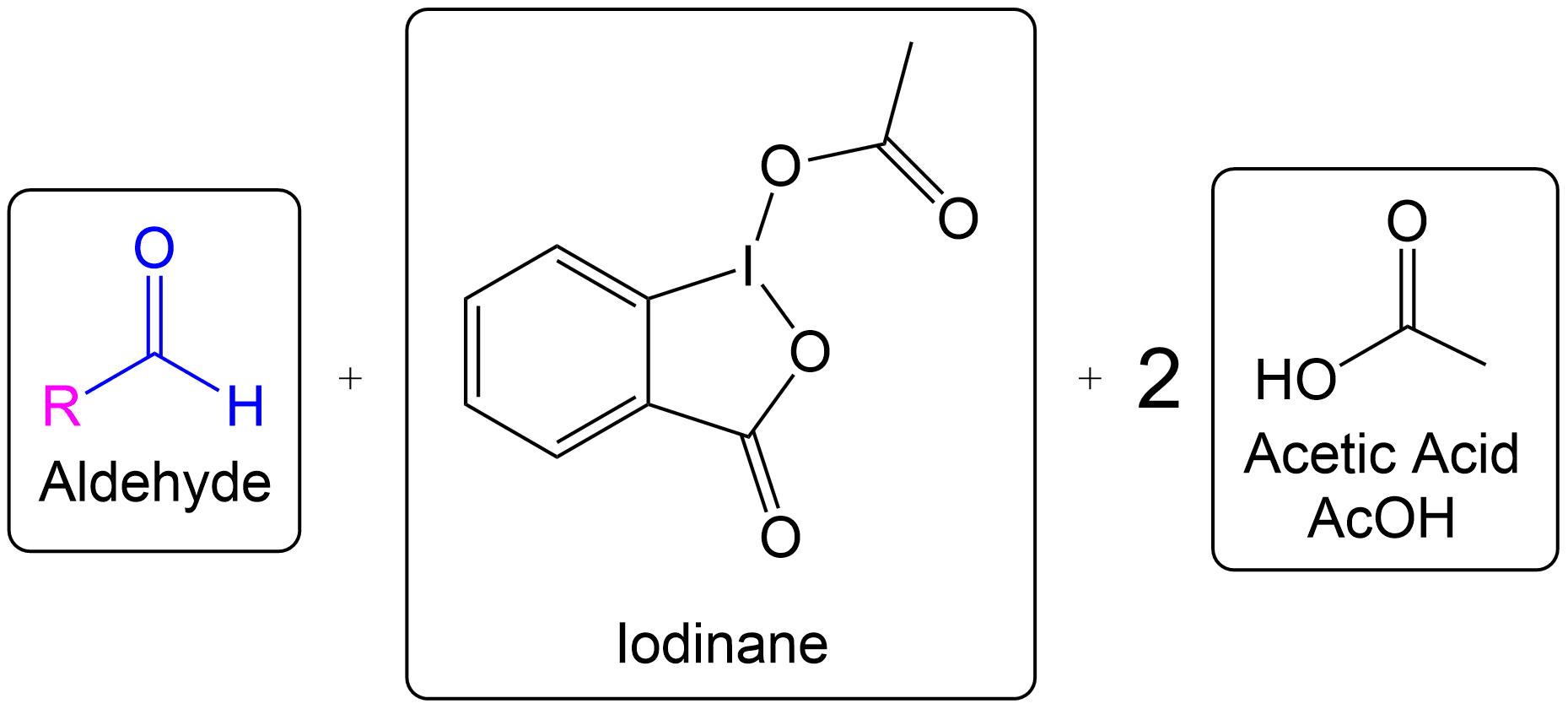
Overall Products recovered post-oxidation.
Formation of the aldehyde product and iodinane and acetic acid as by-products.
See the Shortform Version of the Products
See the Shortform Version of the Products
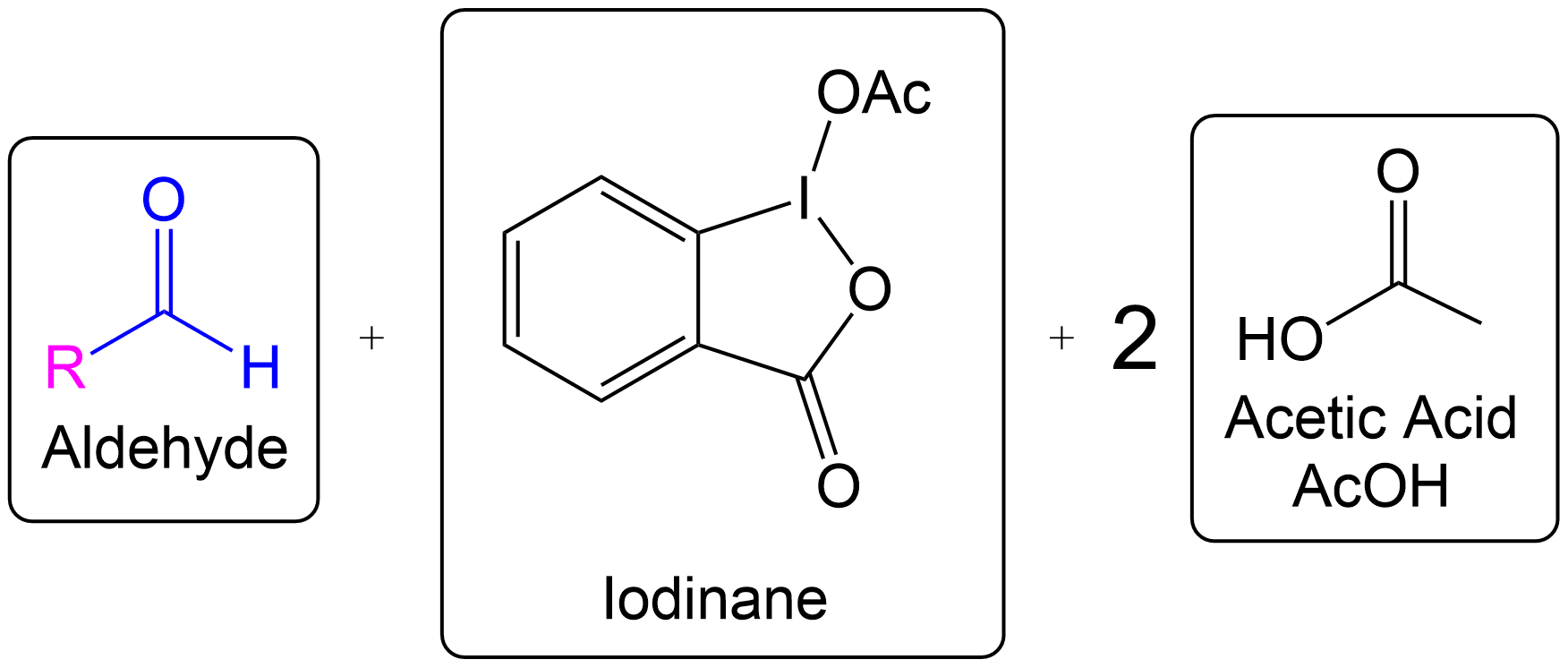
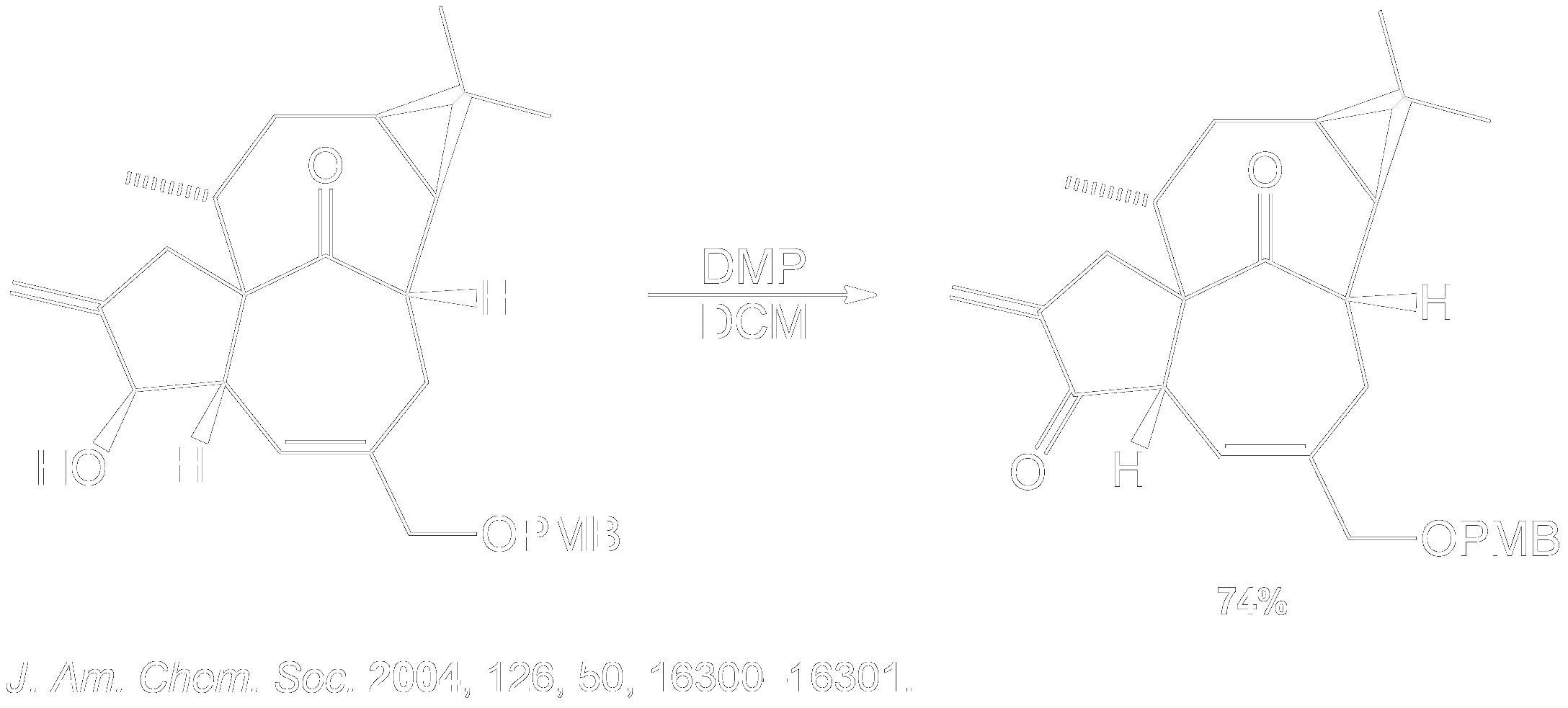
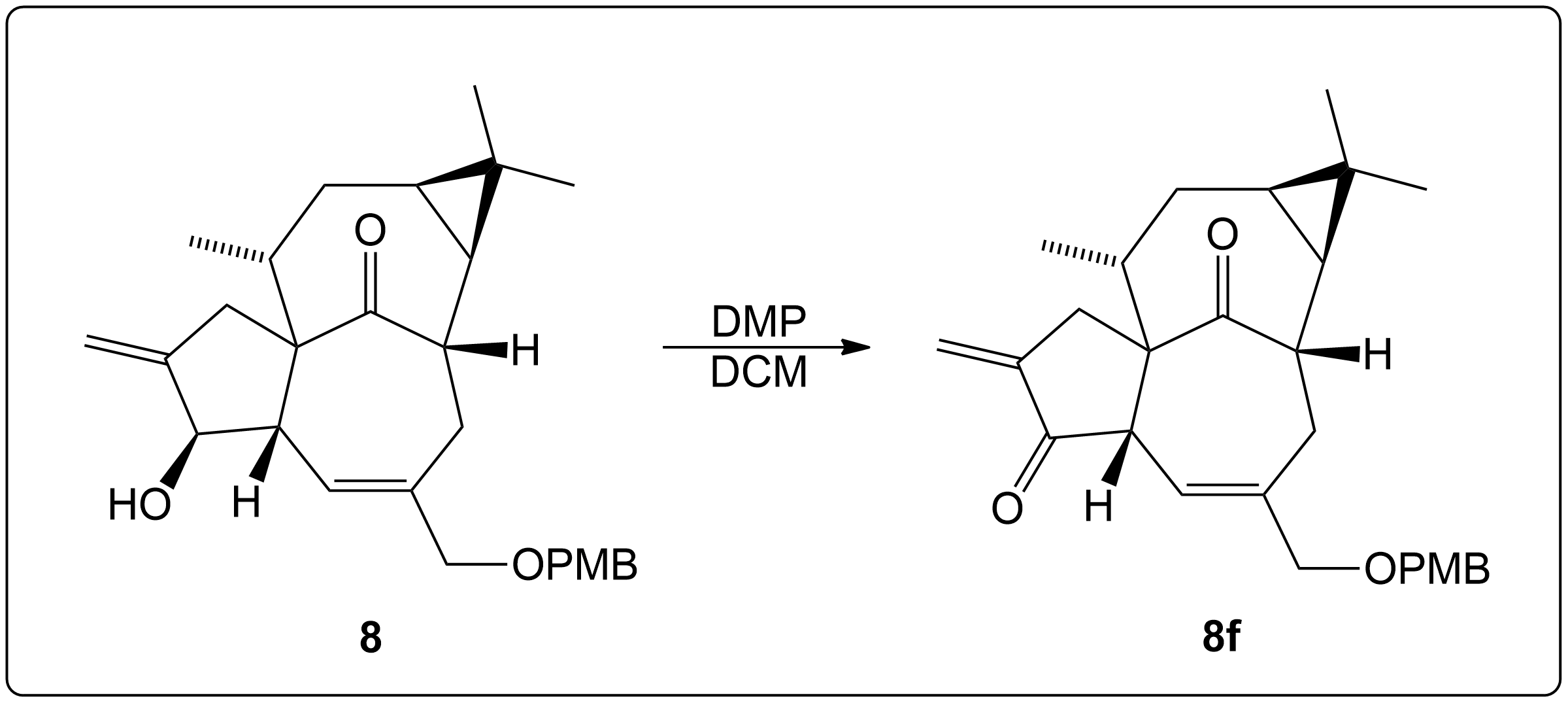
Finding the Product for a 2° Alcohol
This section is a brief overview on how to find the product for a 2° Alcohol (Secondary) using a example from a real scientific research paper.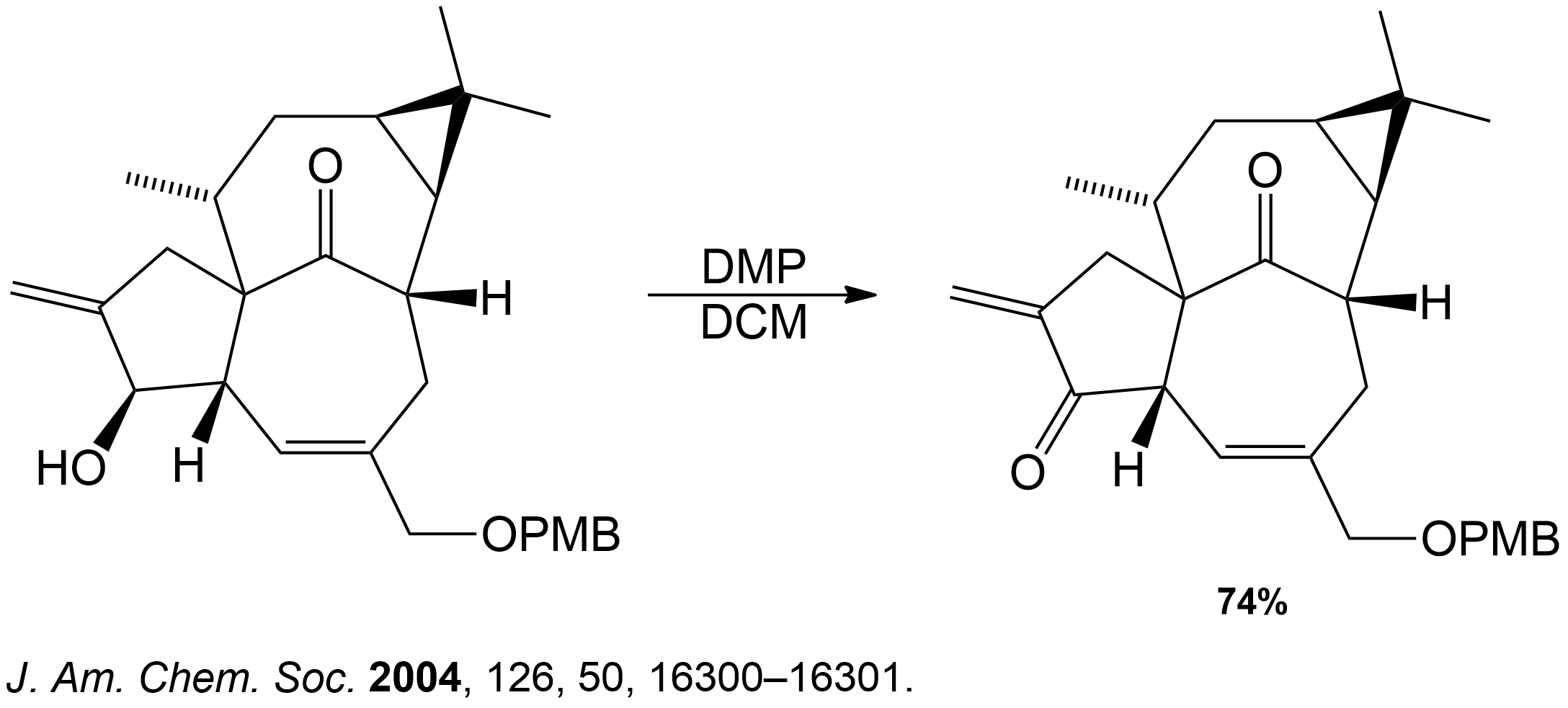
Propose a Mechanism for this Reaction.
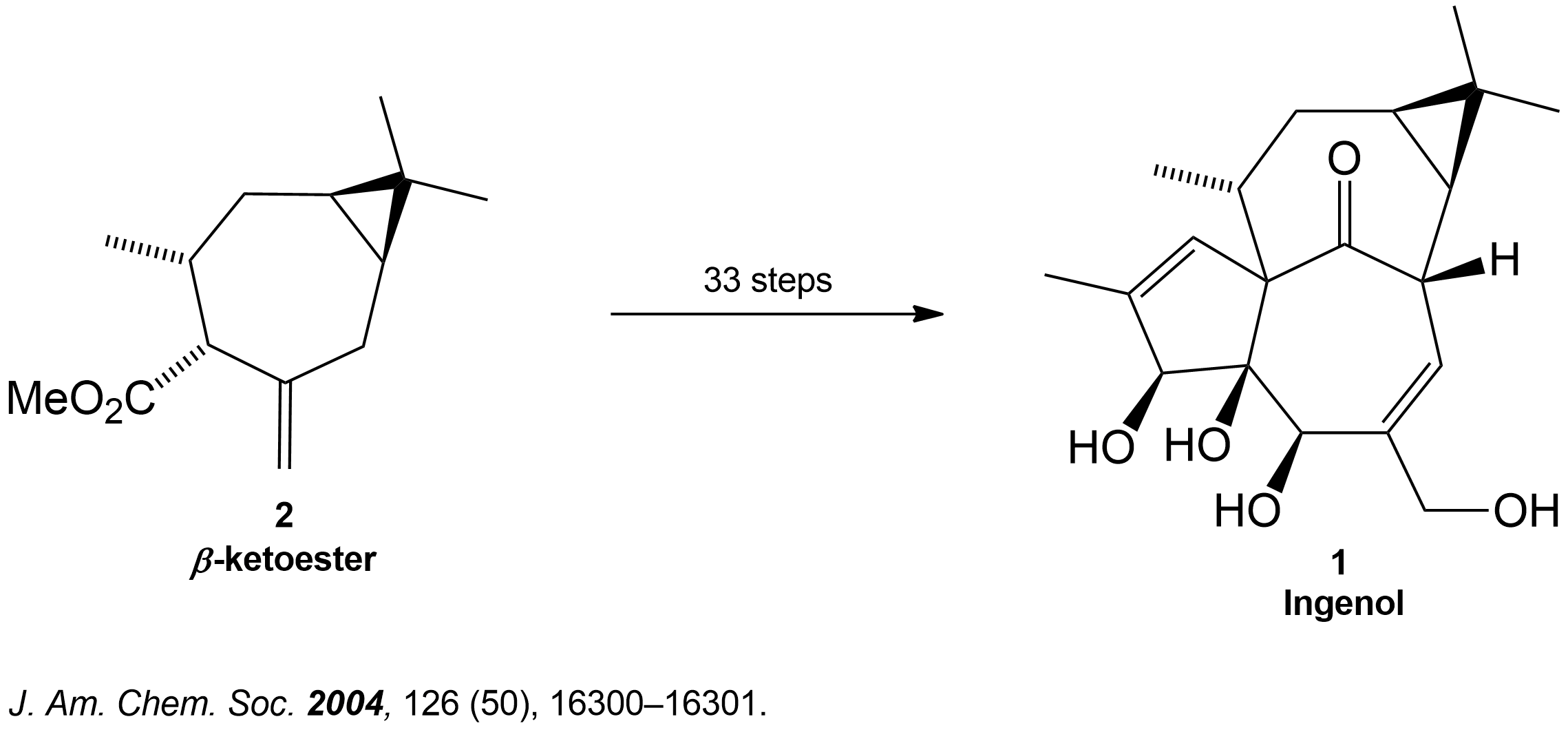
Where did this Reaction come from?
Where did this Reaction come from?
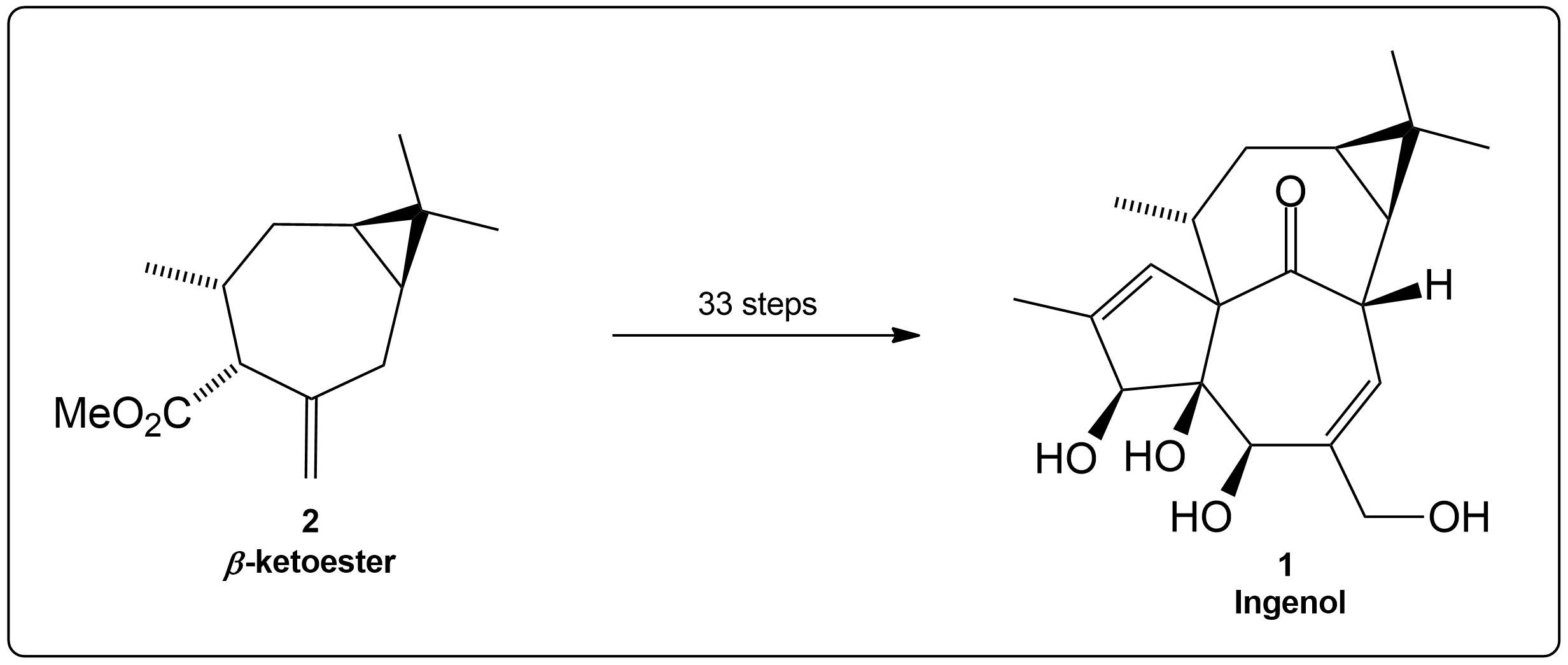
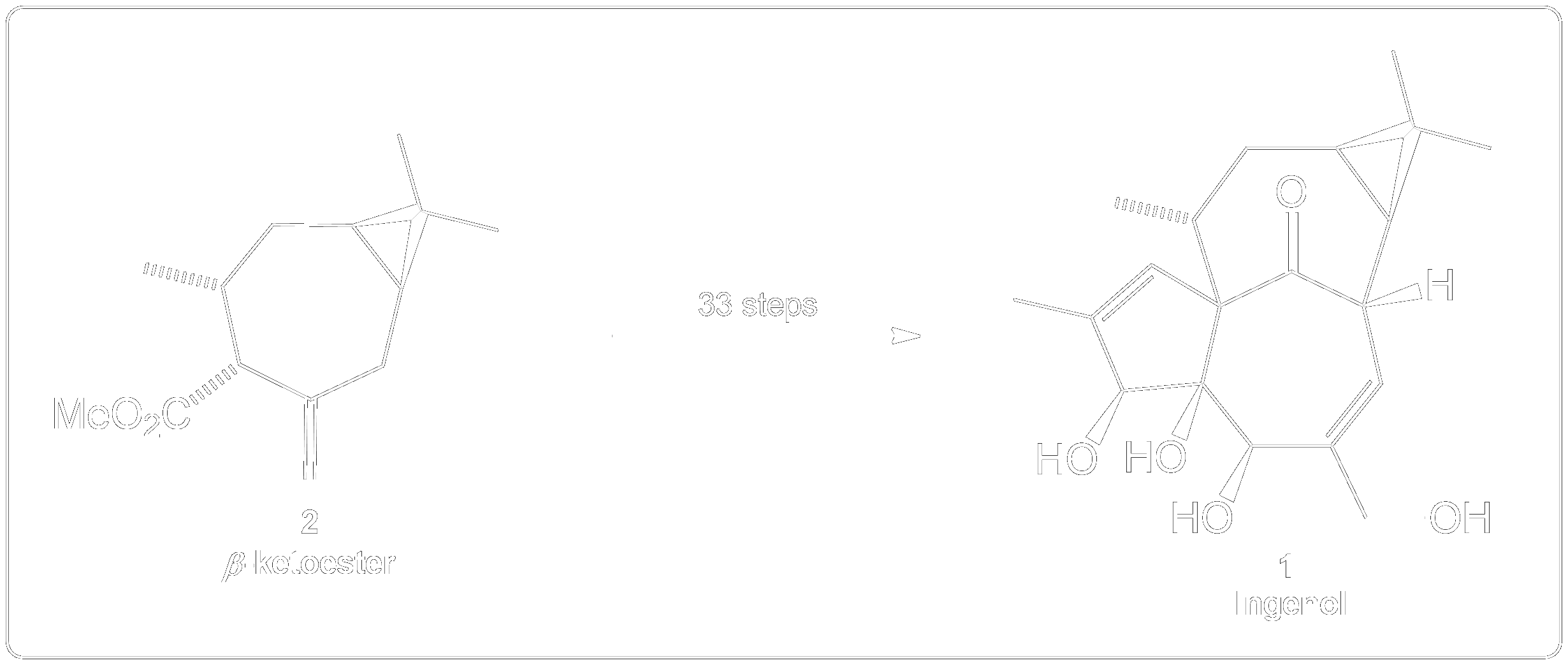
Synthesis Overview of the Total Synthesis of Ingenol.
𝛽-ketoester (2) is subjected through a 32 steps synthesis path to form the target compound Ingenol (1). 3
How is Dess-Martin Oxidation used?
How is Dess-Martin Oxidation used?
Dess-Martin Oxidation Usage in Intermediate Formation.
This reaction showing the Dess-Martin oxidation of the alcohol intermediate was part of the overall synthesis pathway of the formation of Ingenol. Ingenol is the parent compound of several naturally occurring ingenanes with varied peripheral functionalities. These ingenanes display a range of interesting biological profiles, from tumor-promoting to anti-leukemic and anti-HIV activities. 3
Identify the Right Reagents

Identify the Key Features of the Compound

- Primary
- Secondary
- Tertiary
- Primary alcohols can go through Dess-Martin Oxidation to become an Aldehyde.
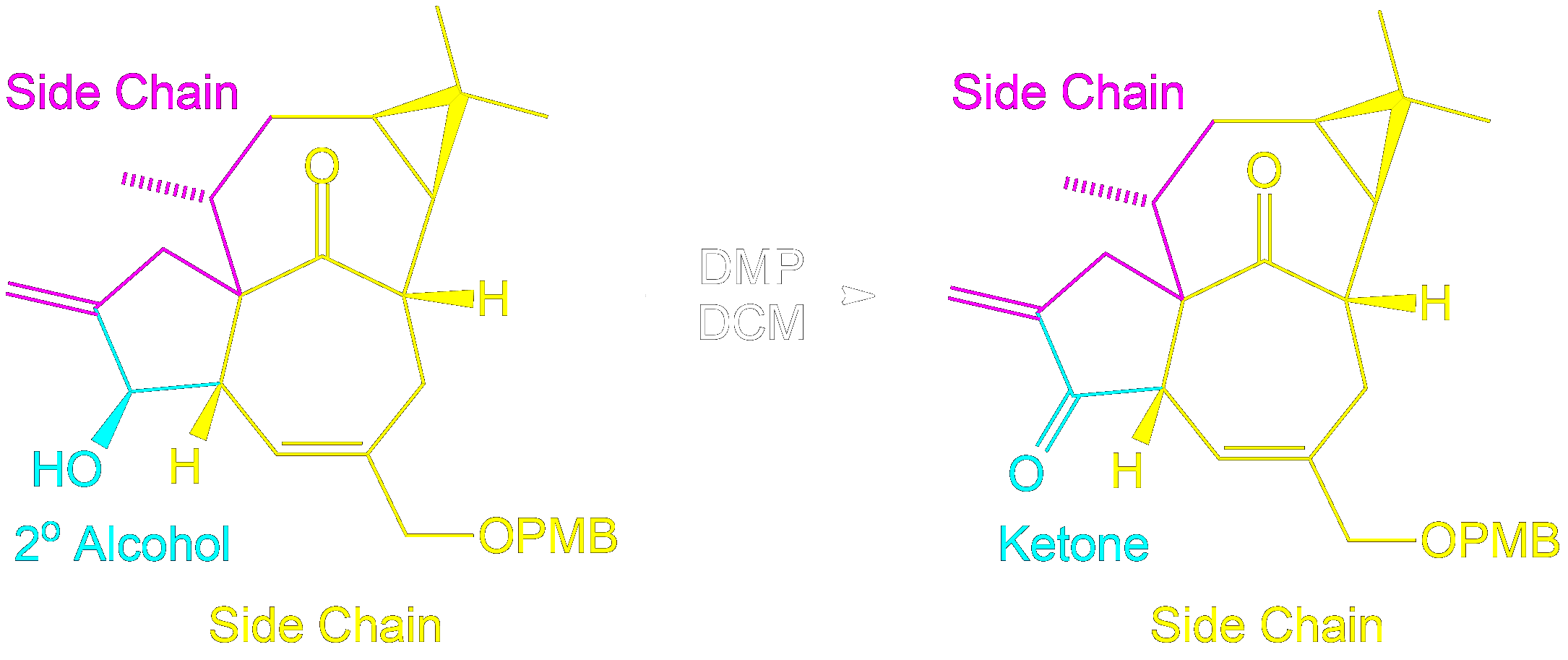
Identifying Side Chains and Alcohol Conversion
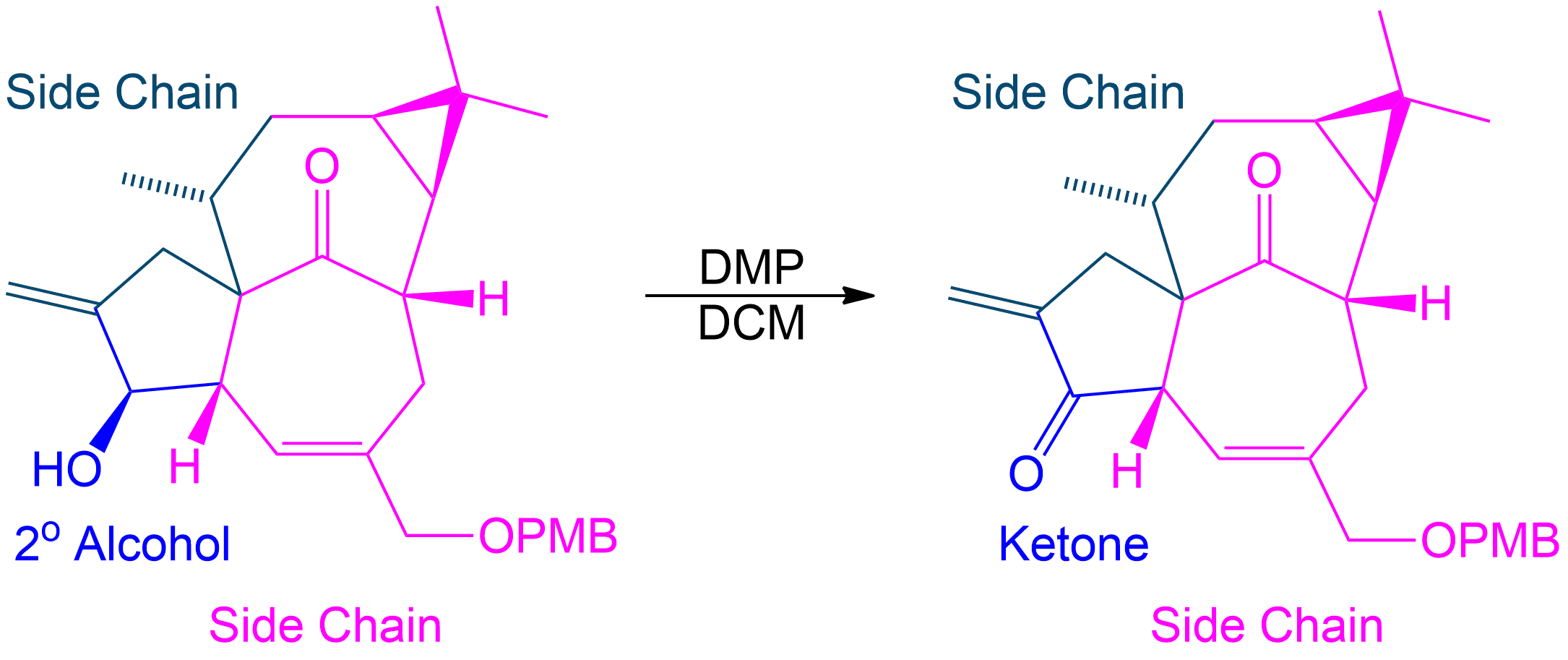
Tracking Side Chains and Alcohol Conversion in Dess-Martin Oxidation
In Dess-Martin oxidation of secondary alcohols, the colored side chains represent R groups that remain unchanged during the reaction.
- Identify Side Chains (R¹ and R²): Use parts of the molecule as placeholders (R¹ and R²), representing parts of the molecule flanking the alcohol group.
- Understand Their Role: These placeholders help track the unchanged parts of the molecule, aiding in visualizing the structure before and after the reaction.
- Focus on the Reaction Center: The secondary alcohol is selectively oxidized to form a ketone. The placeholders show how the structure is altered.
- Reassign Side Chains: After the reaction, reattach the placeholders (R¹ and R²) to the new ketone, demonstrating the unchanged nature of the side chains.
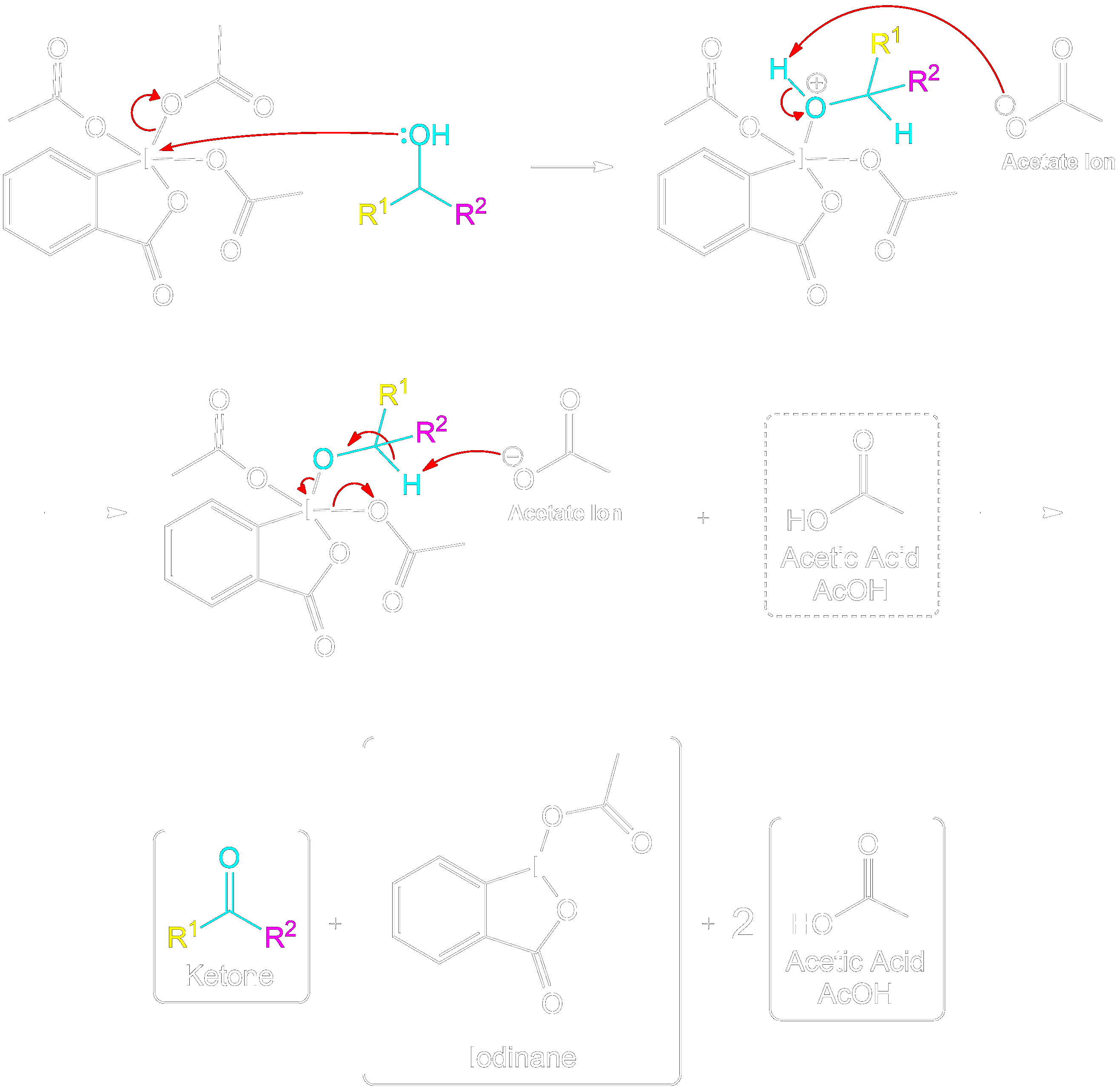
Mechanism for 2° Alcohol
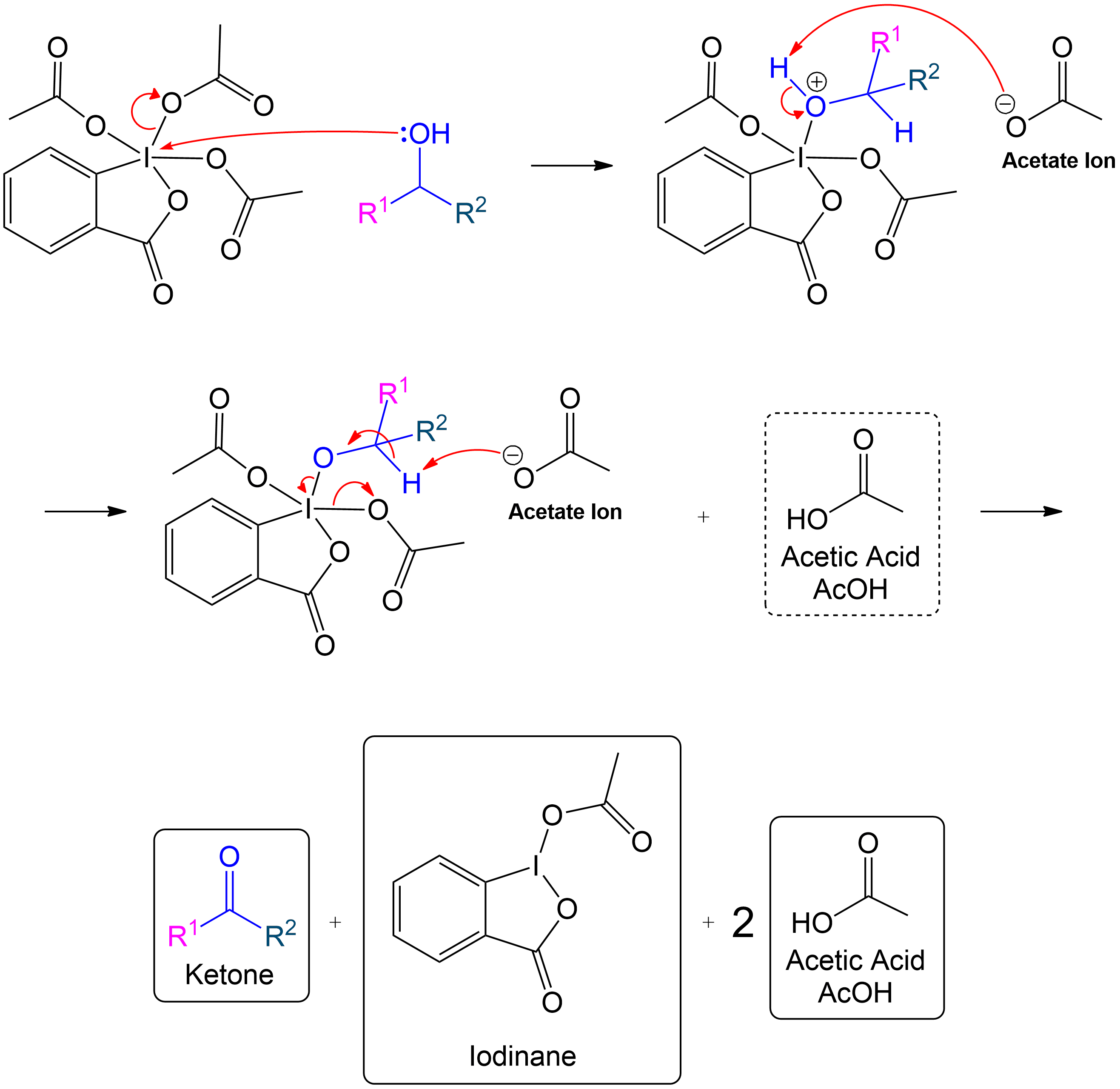
This section is a brief overview on how to perform the mechanism for a 2° Alcohol (Secondary) using the example from above.Reactive Intermediate Formation
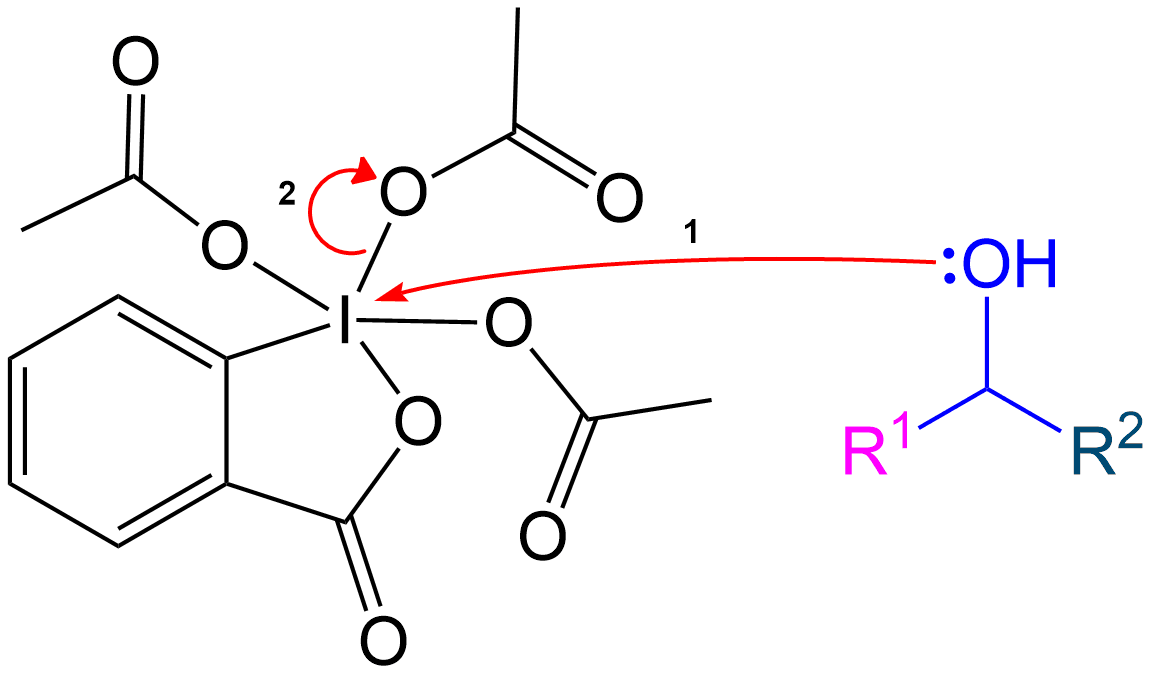
Formation of Reactive Intermediate with Secondary Alcohol
The mechanism follows the same steps as the primary alcohol oxidation but includes an additional side chain.
See the Shortform Version of this Step
See the Shortform Version of this Step
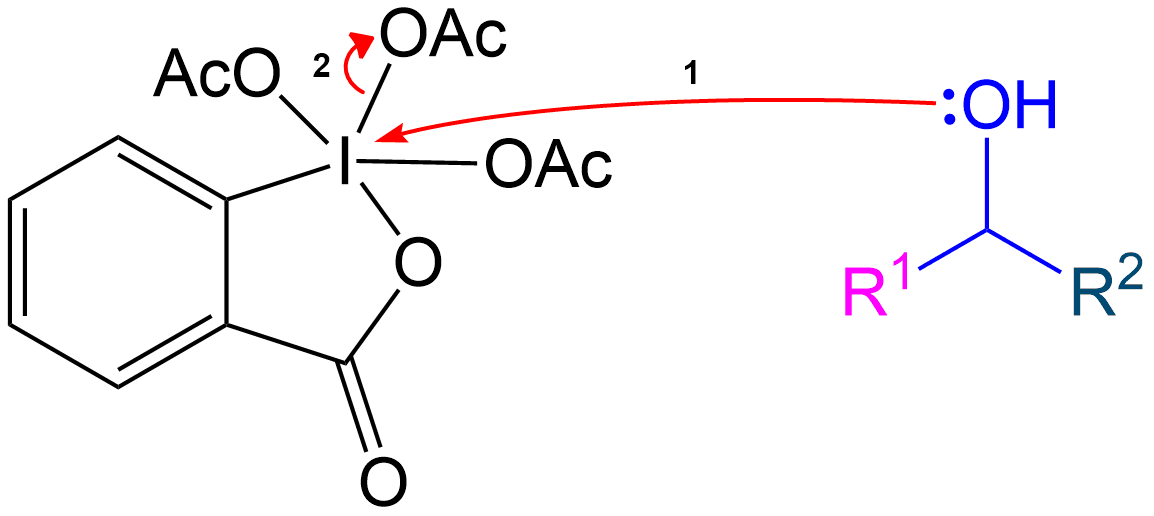
Stabilization with Acetate Ion
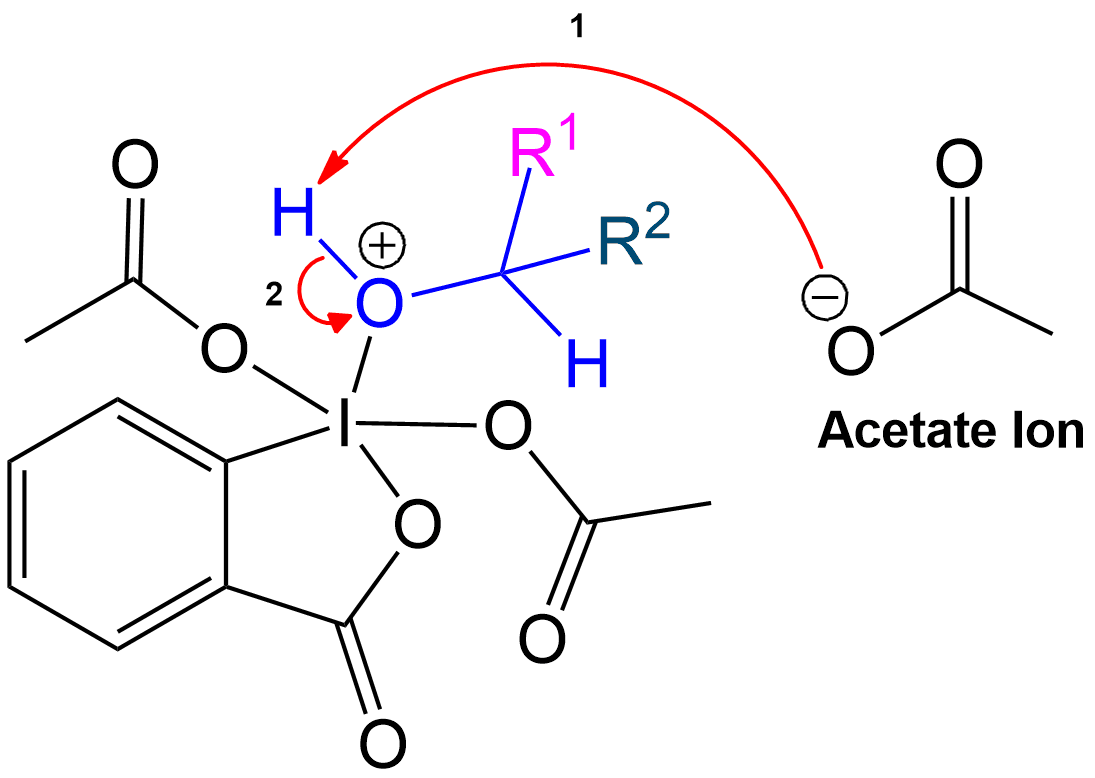
Stabilization of Reactive Intermediate Using an Acetate Ion
The intermediate is stabilized by the expelled OAc group (Acetoxy group).The expelled acetoxy group becomes an acetate ion (CH₃COO⁻).
See the Shortform Version of this Step
See the Shortform Version of this Step
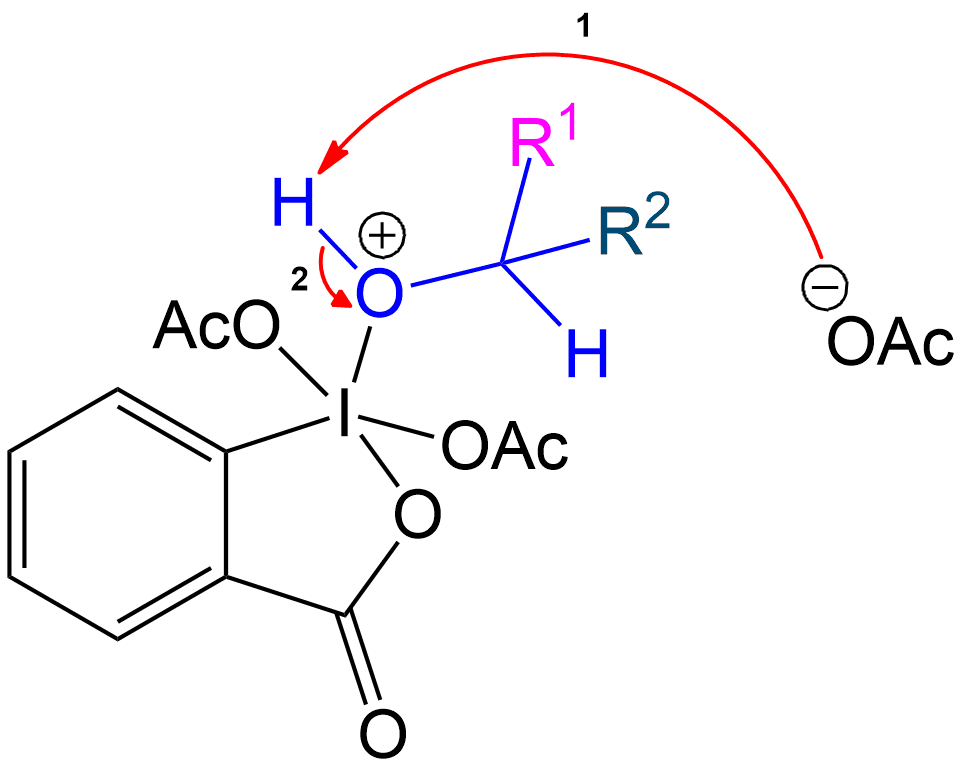
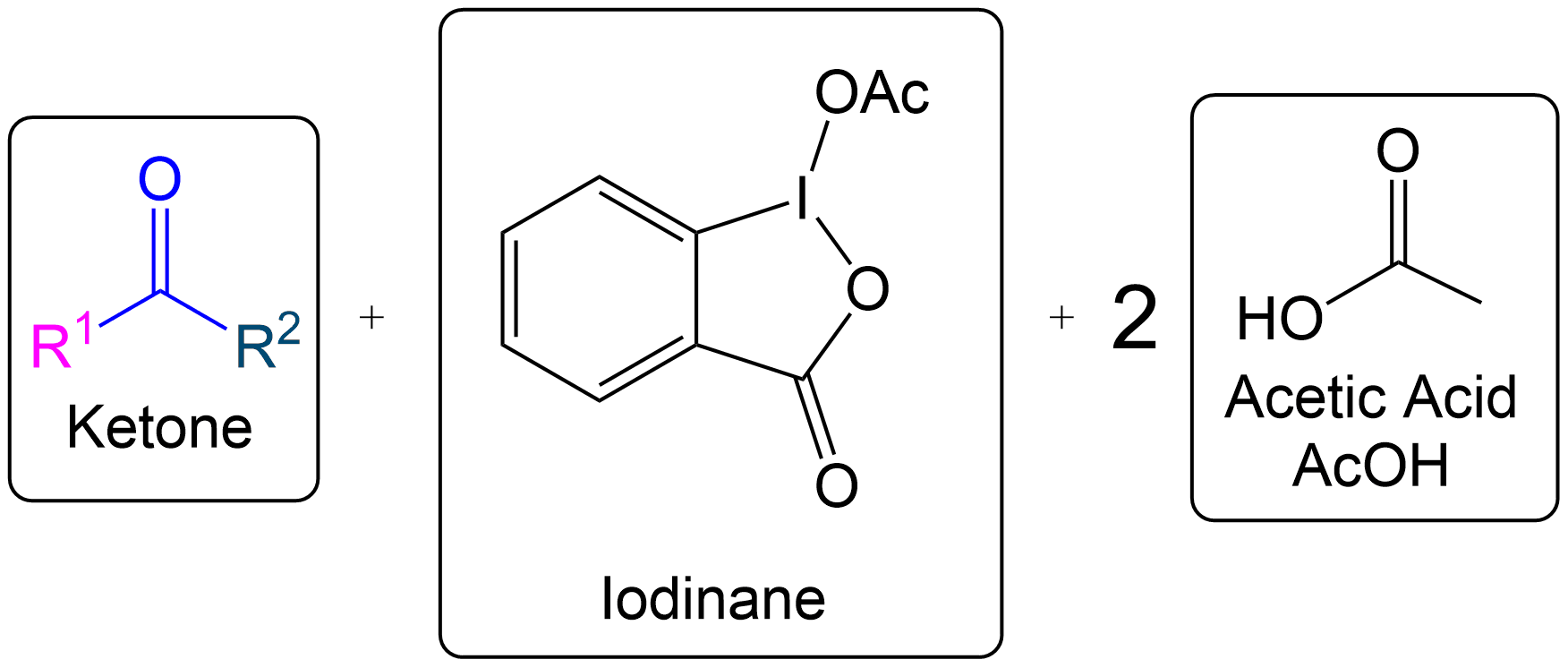
Ketone Formation and Acetic Acid Recovery
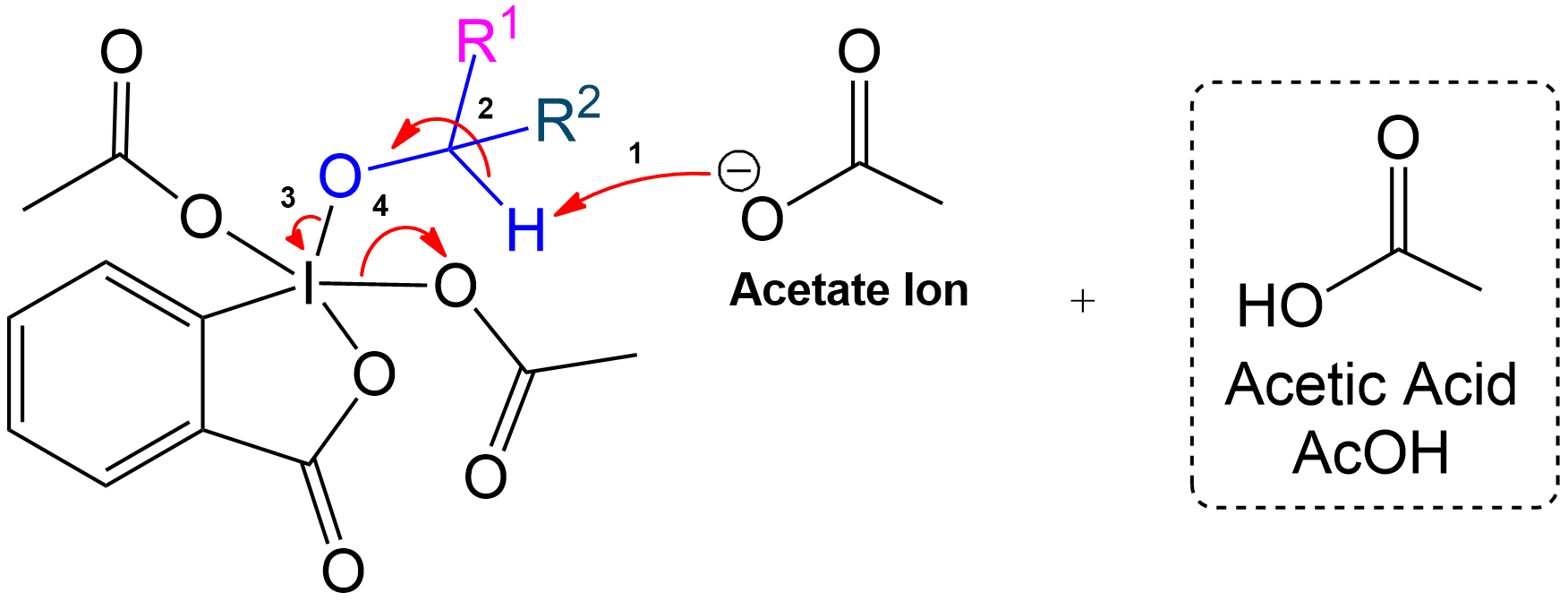
Formation of Ketone Product and Recovery of Acetic Acid.
Acetic acid is recovered, and the intermediate becomes a ketone after proton transfer.
See the Shortform Version of this Step
See the Shortform Version of this Step
Final Overall Products
Final Products of Dess-Martin Oxidation with Secondary Alcohol
The products are similar to those in the primary alcohol oxidation mechanism, with the key difference being the formation of a ketone instead of an aldehyde.
See the Shortform Version of the Products
See the Shortform Version of the Products
Sample Problems
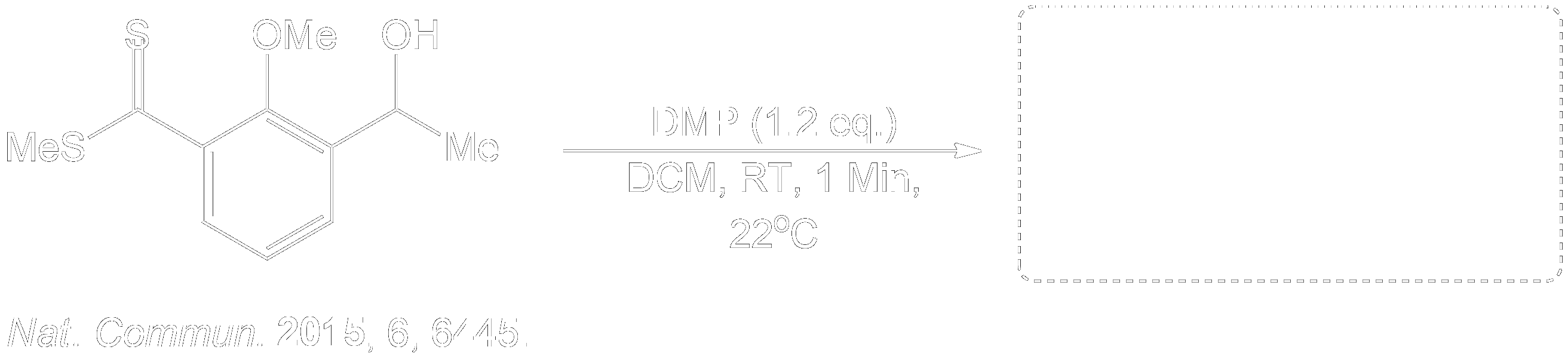
Test your Knowledge.Question 1
Predict the Product.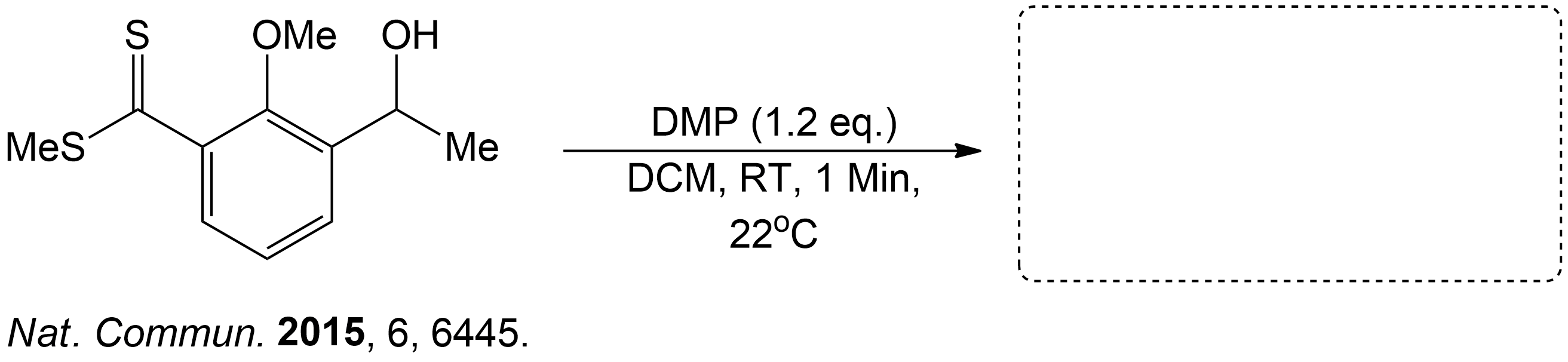
Reveal the Answer.
Reveal the Answer.
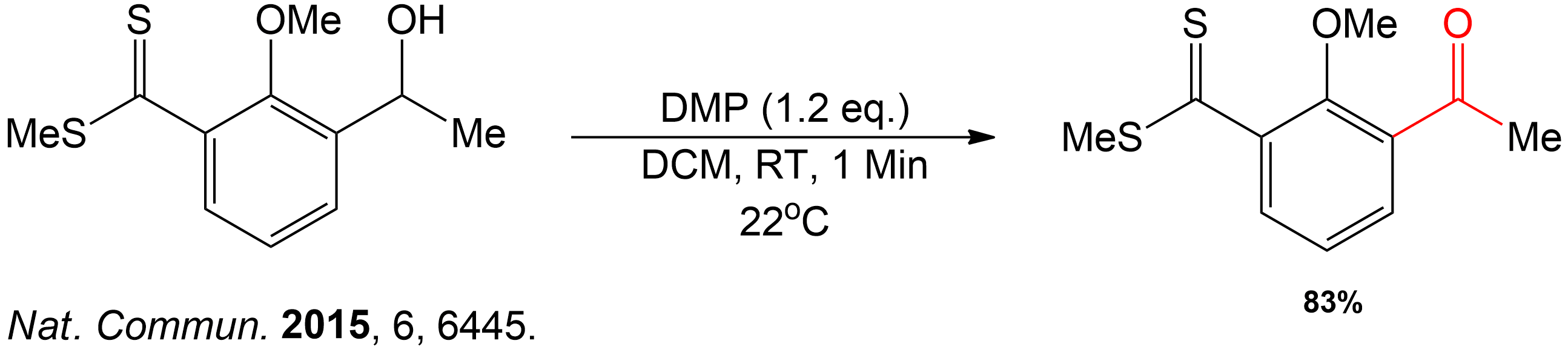
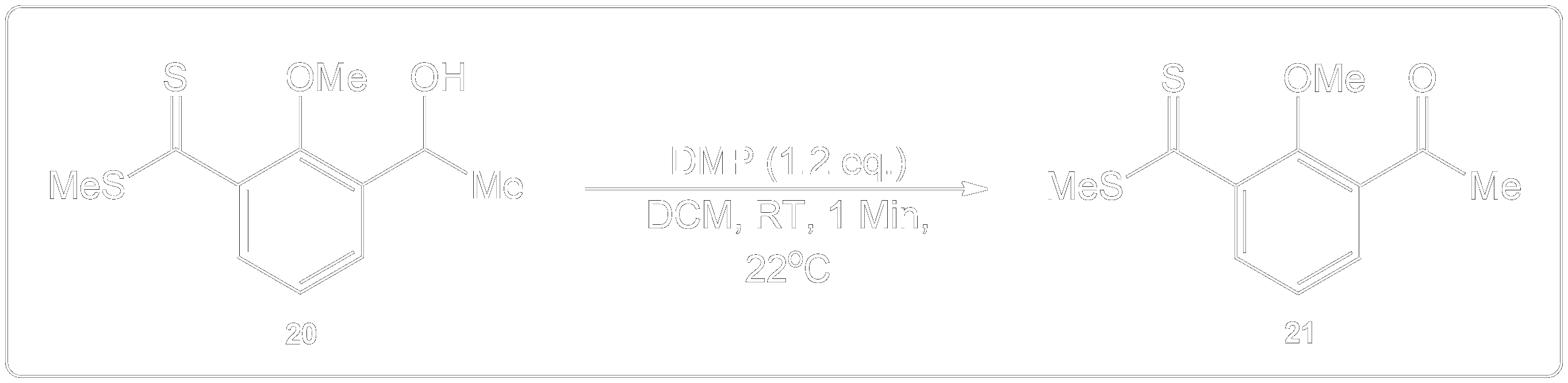
This compound was a secondary alcohol which was oxidized to a ketone.
The alcohol intermediate was oxidized to a ketone using Dess-Martin periodinane (DMP) in dichloromethane (DCM) in room temperature (RT) for 1 minute.
Where did this Reaction come from?
Where did this Reaction come from?
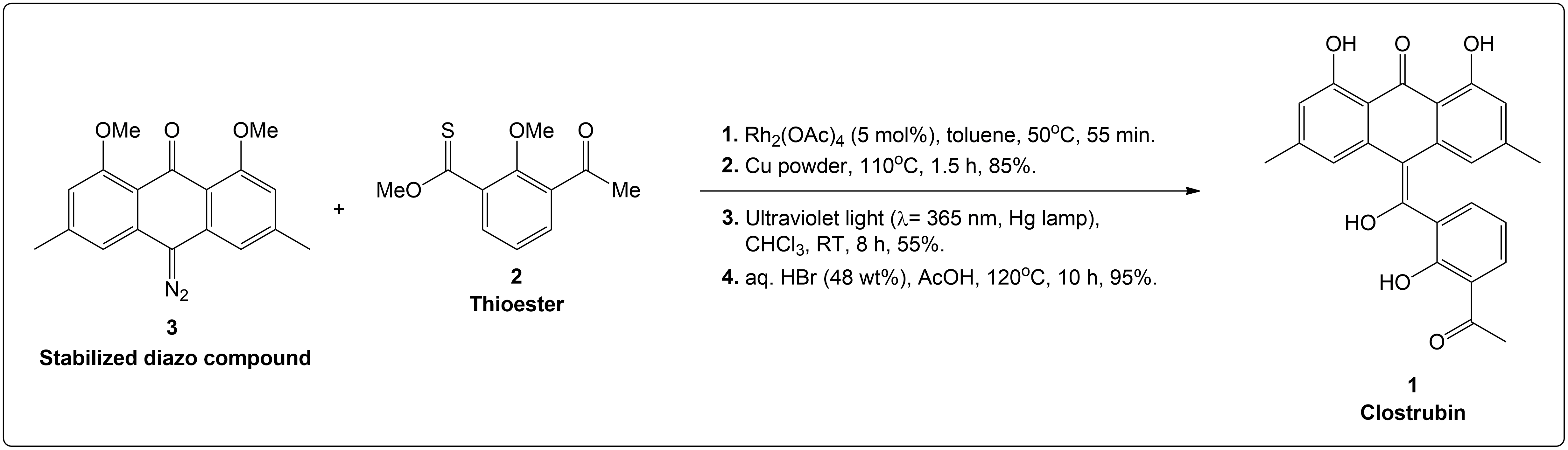
Completion of the synthesis of Clostrubin.
The completion of the Clostrubin (1) synthesis using a stabilized diazo compound and a thioester with several conditions. This caused the formation of a metal-carbenoid intermediate, which was reduced to an episulfide intermediate to a tetrasubstituted olefin, followed by ultraviolet light-induced electrocyclization and final global deprotection, which yielded the desired natural product efficiently.
How is Dess-Martin Oxidation used?
How is Dess-Martin Oxidation used?
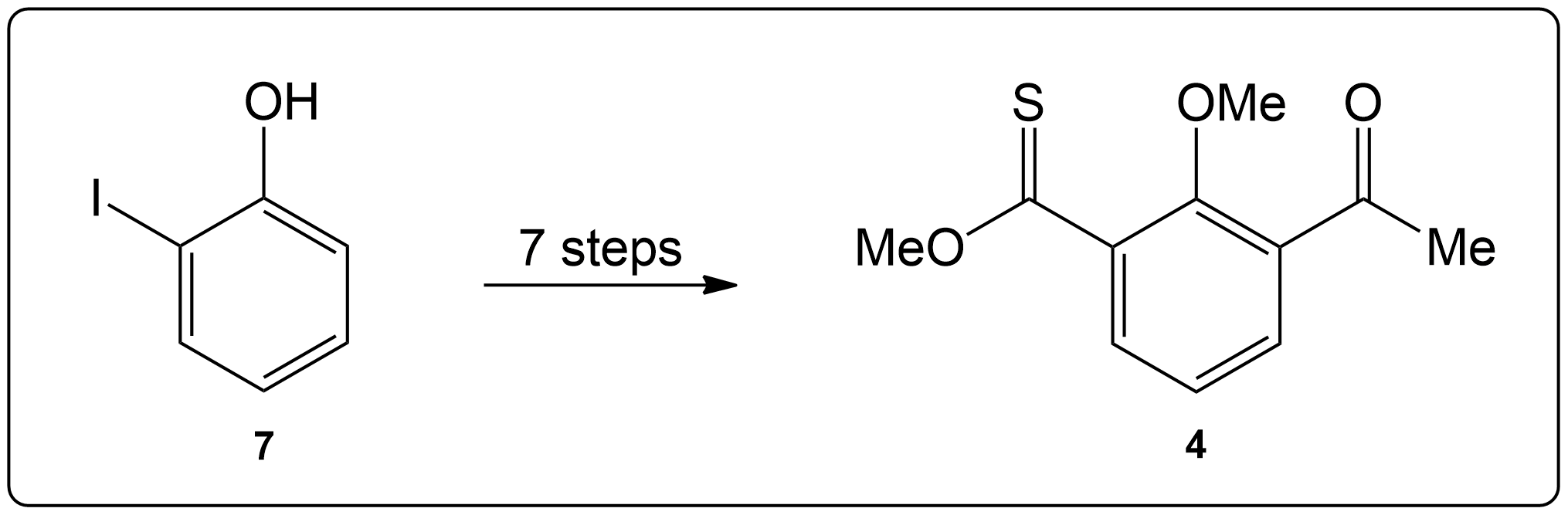
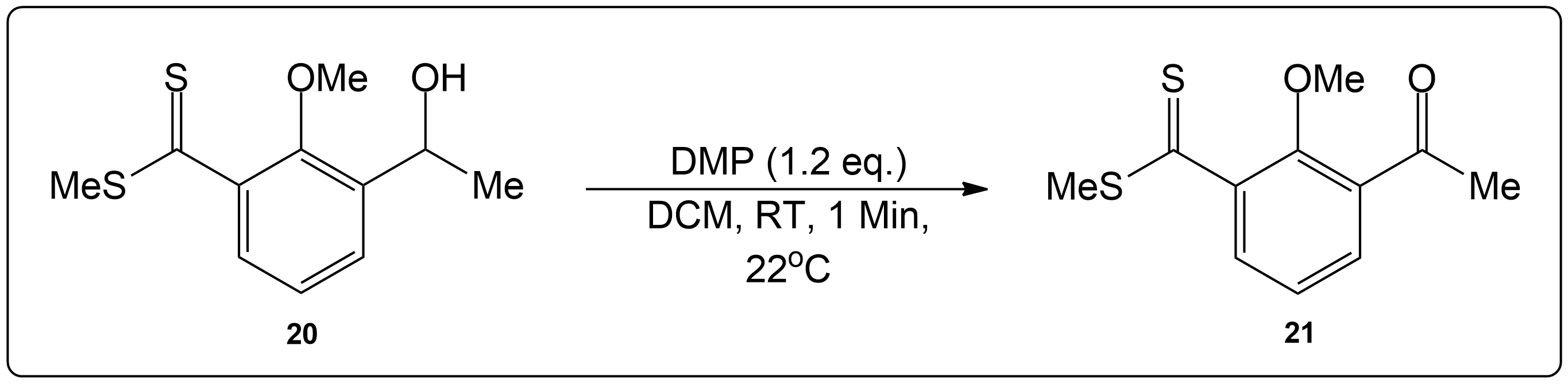
Question 2
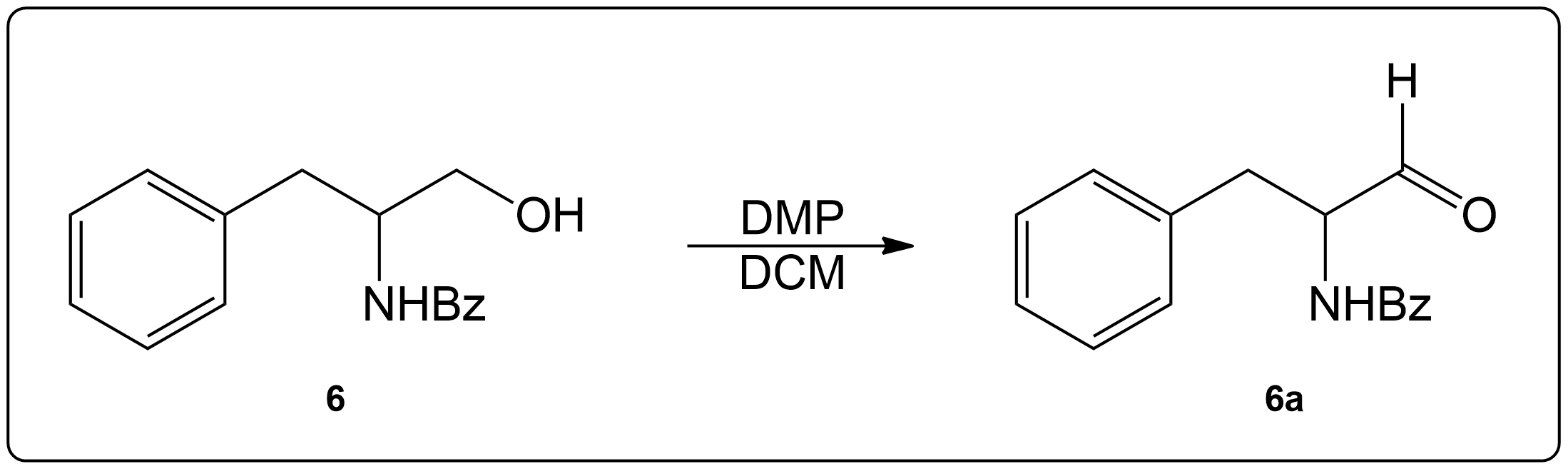
Propose a Mechanism for this Reaction.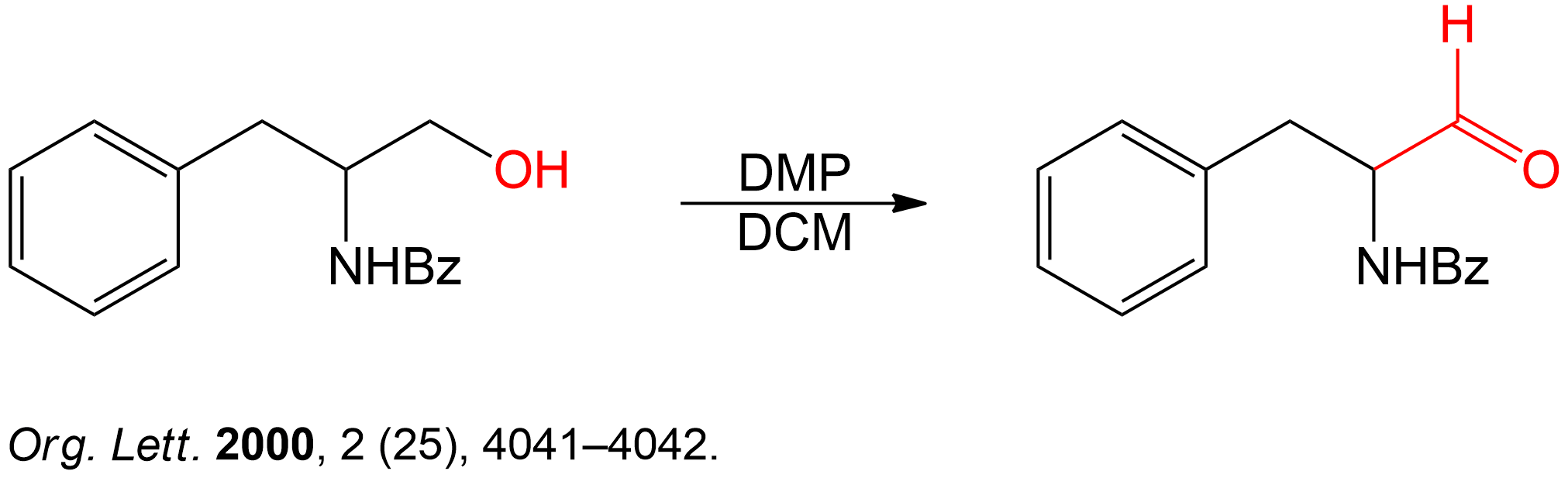
Reveal the Answer.
Reveal the Answer.
Identify the necessary side chain and product.
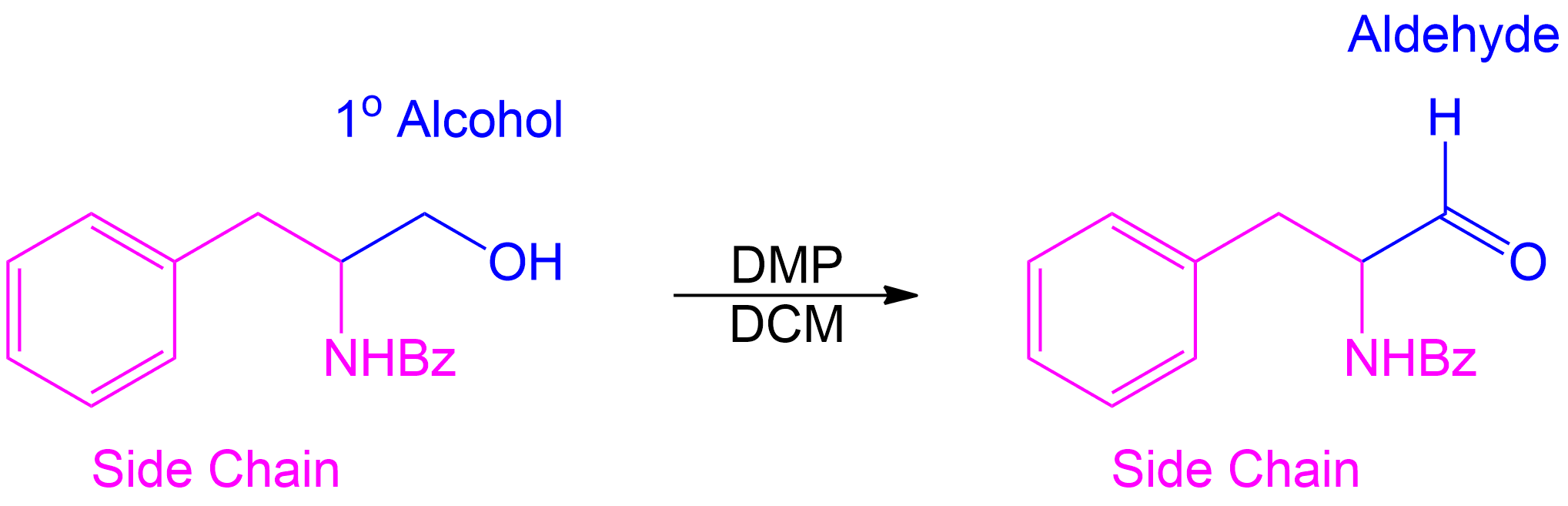
This compound was a primary alcohol.
Start by identifying the non-alcohol portion of the molecule and determine the product.
Where did this Reaction come from?
Where did this Reaction come from?
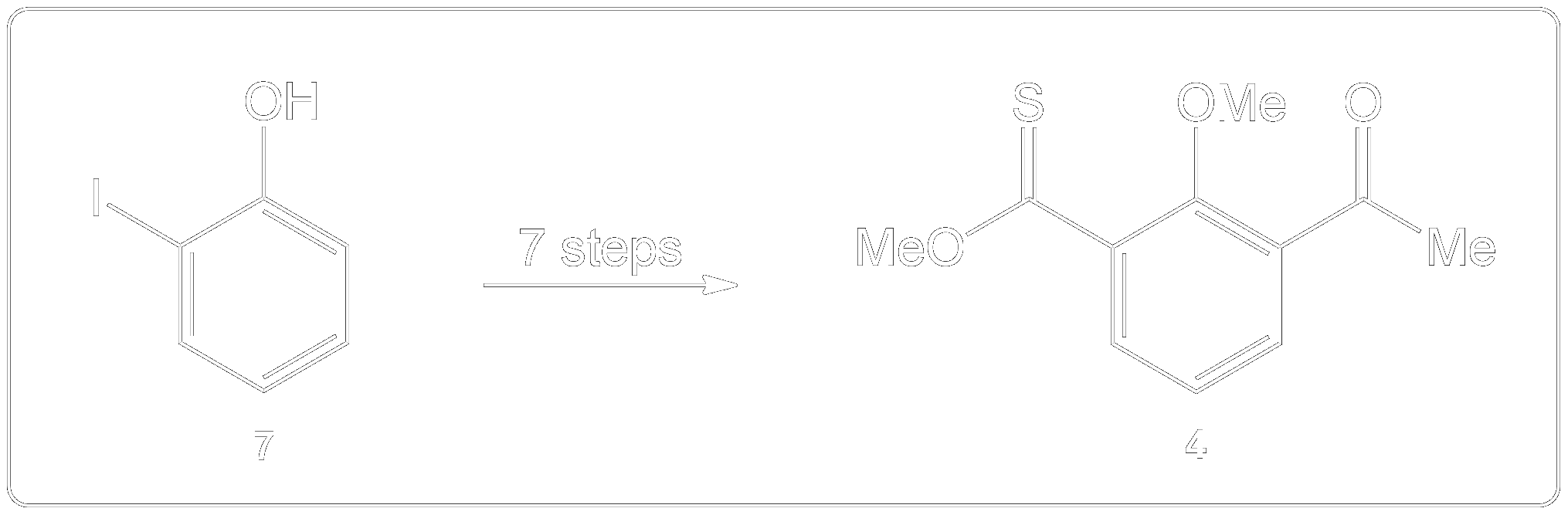
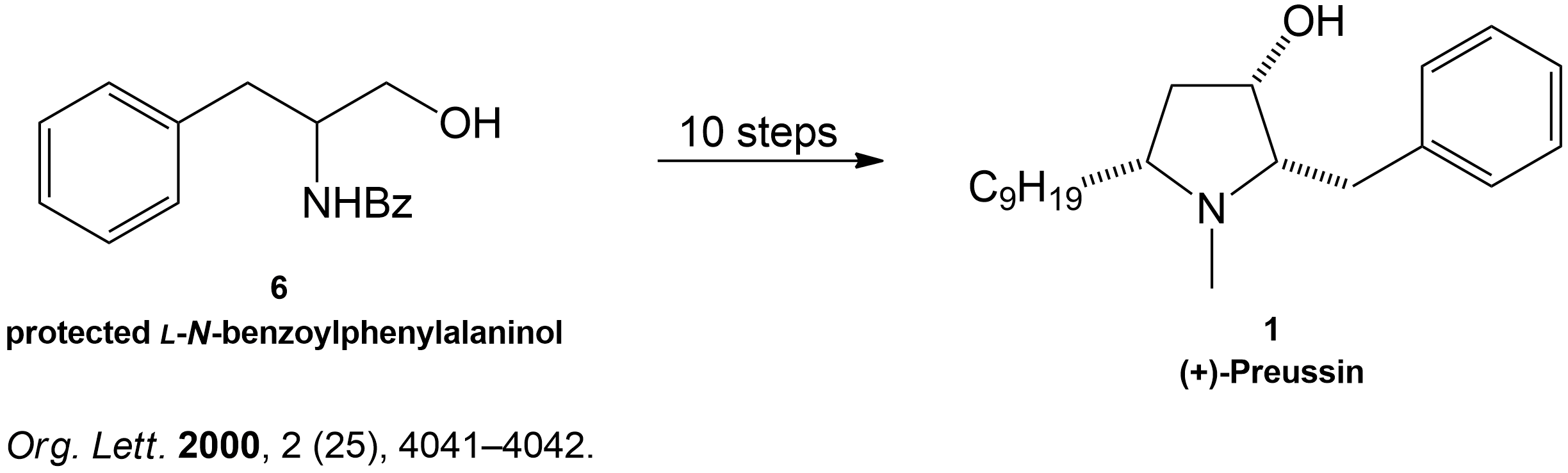
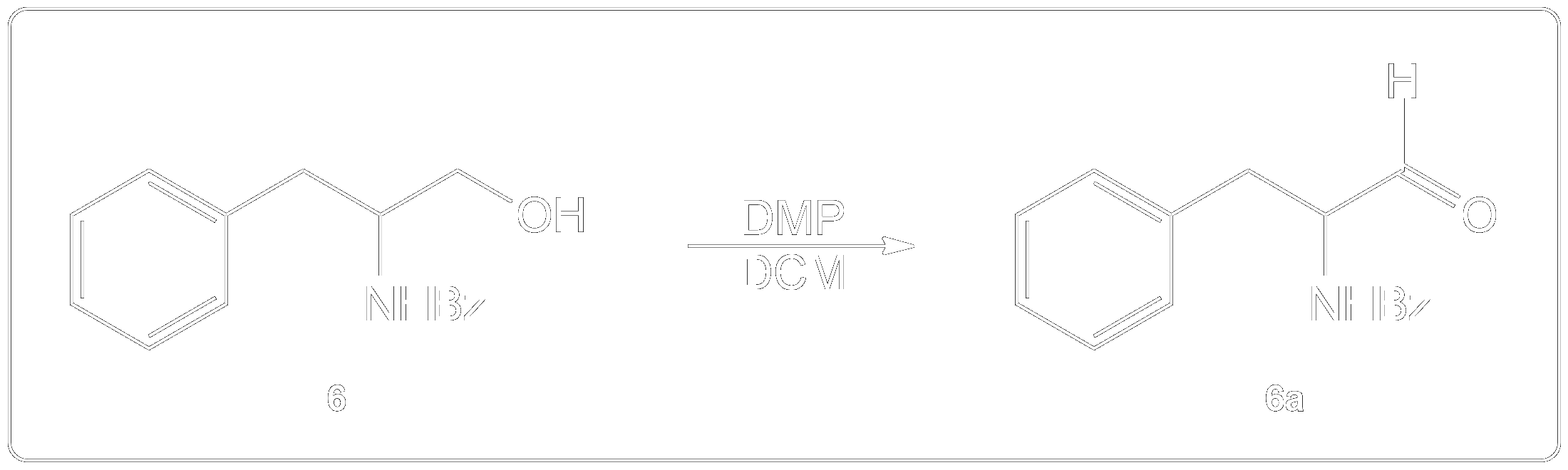
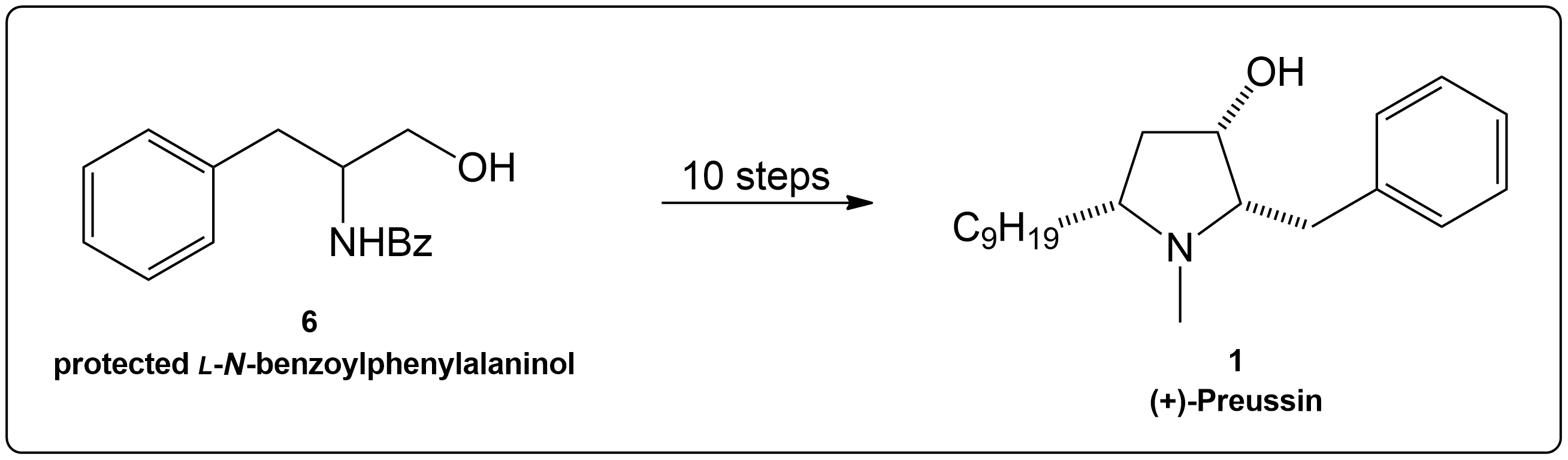
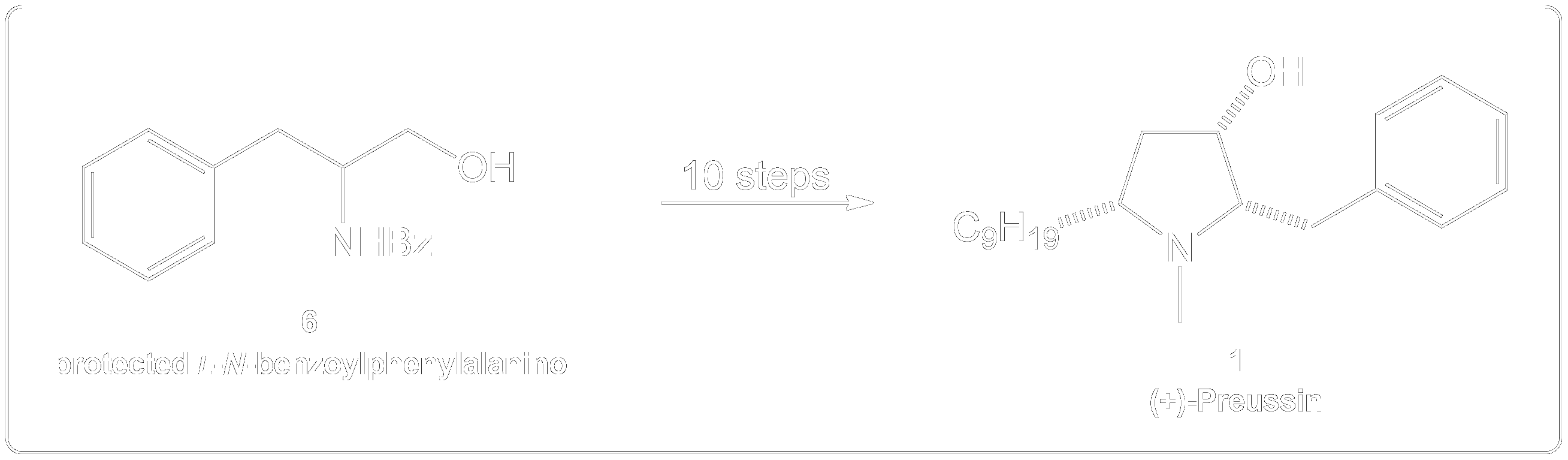
Completion of the synthesis of (+)-Preussin.
Scheme for the enantioselective total synthesis of (+)-Preussin (1) from protected L-N-benzoylphenylalaninol (6) over 10 steps.
How is Dess-Martin Oxidation used?
How is Dess-Martin Oxidation used?
Summary
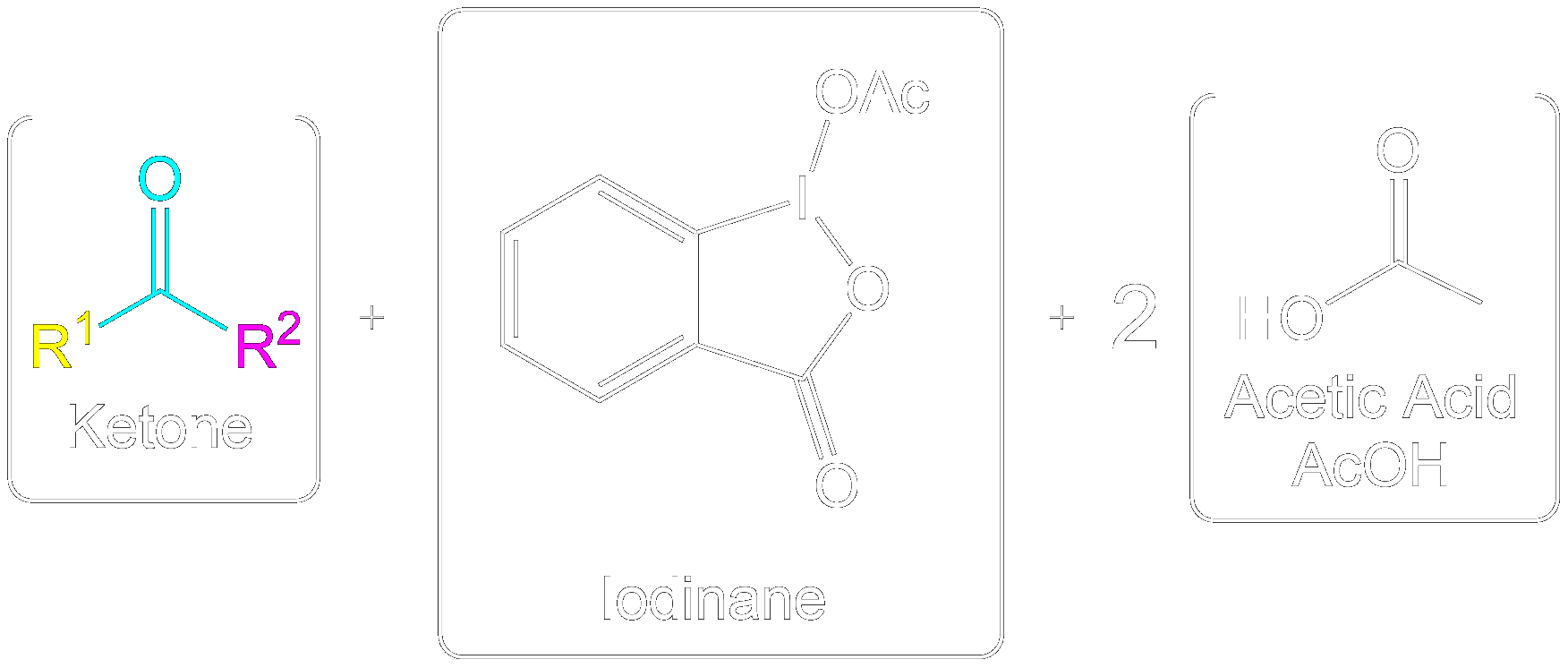
The reaction entry summary. Find the general scheme and full summarized mechanisms here.General Scheme
This section briefly summarizes what can and cannot undergo reactions.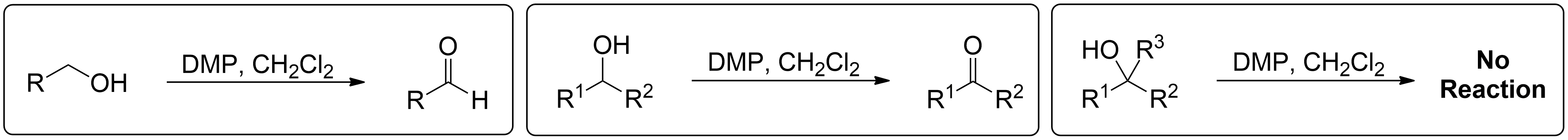
- 1° Alcohols (Primary) get oxidized to Aldehydes.
- 2° Alcohols (Secondary) get oxidized to Ketones.
- 3° Alcohols (Tertiary) do not get oxidized at all.
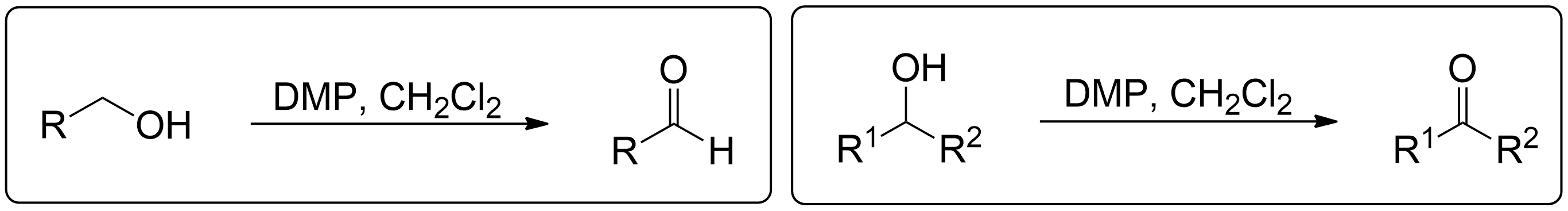
General Mechanism
This section briefly summarizes steps to find the product and perform the mechanisms. Quick steps to finding the product for any alcohol- Identify the reagents.
- Assign side chains (non alcohol part).
- Selectively convert Alcohol to correct product based on alcohol type. Nothing else.
- Keep the side chains (non alcohol part) the same and piece together the full molecule together again.
Full Primary Mechanism
Full Primary Mechanism
Full Secondary Mechanism
Full Secondary Mechanism
References
- Dess, D. B.; Martin, J. C. Readily Accessible 12-I-51 Oxidant for the Conversion of Primary and Secondary Alcohols to Aldehydes and Ketones. J. Org. Chem. 1983, 48, 4155–4156. DOI: 10.1021/jo00170a070
- Lee, H. M.; Nieto-Oberhuber, C.; Shair, M. D. Enantioselective synthesis of (+)-cortistatin A, a potent and selective inhibitor of endothelial cell proliferation. J. Am. Chem. Soc. 2008, 130 (50), 16864–16866. DOI: 10.1021/ja8071918
- Nickel, A.; Maruyama, T.; Tang, H.; Murphy, P. D.; Greene, B.; Yusuff, N.; Wood, J. L. Total synthesis of ingenol. J. Am. Chem. Soc. 2004, 126 (50), 16300–16301. DOI: 10.1021/ja044123l
- Yang, M.; Li, J.; Li, A. Total synthesis of clostrubin. Nat. Commun. 2015, 6, 6445. DOI: 10.1038/ncomms7445
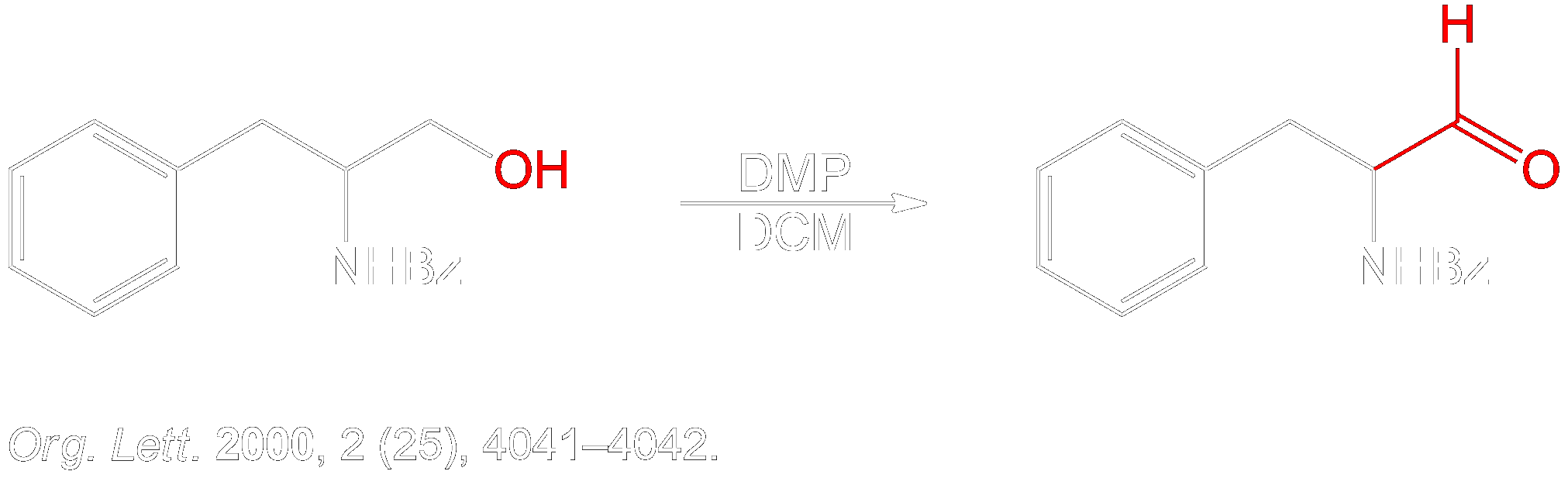
- Lee, K.-Y.; Kim, Y.-H.; Oh, C.-Y.; Ham, W.-H. Facile and efficient total synthesis of (+)-preussin. Org. Lett. 2000, 2 (25), 4041–4042. DOI: 10.1021/ol000289p